Monoamine neurotransmitter
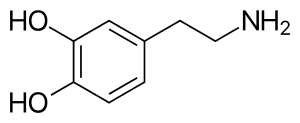
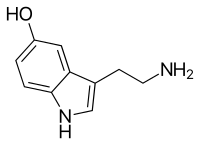
Monoamine neurotransmitters are neurotransmitters and neuromodulators that contain one amino group connected to an aromatic ring by a two-carbon chain (such as -CH2-CH2-). Examples are dopamine, norepinephrine and serotonin.
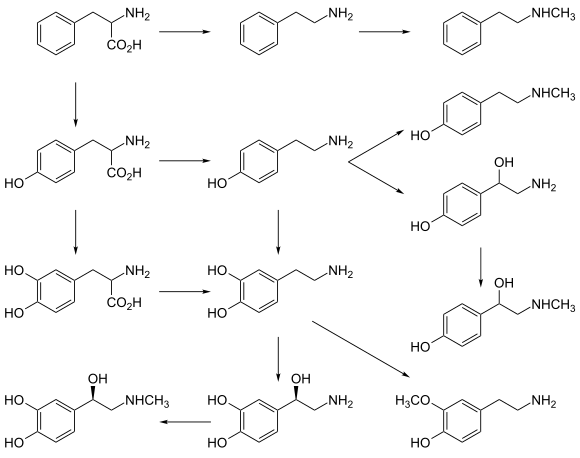
All monoamines are derived from aromatic amino acids like phenylalanine, tyrosine, and tryptophan by the action of aromatic amino acid decarboxylase enzymes. They are deactivated in the body by the enzymes known as monoamine oxidases which clip off the amine group.
Monoaminergic systems, i.e., the networks of neurons that use monoamine neurotransmitters, are involved in the regulation of processes such as emotion, arousal, and certain types of memory. It has also been found that monoamine neurotransmitters play an important role in the secretion and production of neurotrophin-3 by astrocytes, a chemical which maintains neuron integrity and provides neurons with trophic support.[1]
Drugs used to increase or reduce the effect of monoamine neurotransmitters are used to treat patients with psychiatric and neurological disorders, including depression, anxiety, schizophrenia and Parkinson's disease.[2]
Examples
- Classical monoamines
- Imidazoleamines:
- Catecholamines:
- Adrenaline (Ad; Epinephrine, Epi)
- Dopamine (DA)
- Noradrenaline (NAd; Norepinephrine, NE)
- Indolamines:
- Trace amines
- Phenethylamines (related to catecholamines):
- Tryptamine[10][8][9]
Specific transporter proteins called monoamine transporters that transport monoamines in or out of a cell exist. These are the dopamine transporter (DAT), serotonin transporter (SERT), and the norepinephrine transporter (NET) in the outer cell membrane and the vesicular monoamine transporter (VMAT1 and VMAT2) in the membrane of intracellular vesicles.
After release into the synaptic cleft, monoamine neurotransmitter action is ended by reuptake into the presynaptic terminal. There, they can be repackaged into synaptic vesicles or degraded by the enzyme monoamine oxidase (MAO), which is a target of monoamine oxidase inhibitors, a class of antidepressants.
Evolution
Monoamine neurotransmitter systems occur in virtually all vertebrates, where the evolvability of these systems has served to promote the adaptability of vertebrate species to different environments.[12][13]
A recent computational investigation of genetic origins shows that the earliest development of monoamines occurred 650 million years ago and that the appearance of these chemical, necessary for active or participatory awareness a and engagement with the environment, coincides with the emergence of bilaterian or “mirror” body in the midst of (or perhaps in some sense catalytic of?) the Cambrian Explosion.[14]
See also
References
- Mele, Tina; Čarman-Kržan, Marija; Jurič, Damijana Mojca (2010). "Regulatory role of monoamine neurotransmitters in astrocytic NT-3 synthesis". International Journal of Developmental Neuroscience. 28 (1): 13–9. doi:10.1016/j.ijdevneu.2009.10.003. PMID 19854260. S2CID 25734591.
- Kurian, Manju A; Gissen, Paul; Smith, Martin; Heales, Simon JR; Clayton, Peter T (2011). "The monoamine neurotransmitter disorders: An expanding range of neurological syndromes". The Lancet Neurology. 10 (8): 721–33. doi:10.1016/S1474-4422(11)70141-7. PMID 21777827. S2CID 32271477.
- Broadley KJ (March 2010). "The vascular effects of trace amines and amphetamines". Pharmacology & Therapeutics. 125 (3): 363–375. doi:10.1016/j.pharmthera.2009.11.005. PMID 19948186.
- Lindemann L, Hoener MC (May 2005). "A renaissance in trace amines inspired by a novel GPCR family". Trends in Pharmacological Sciences. 26 (5): 274–281. doi:10.1016/j.tips.2005.03.007. PMID 15860375.
- Wang X, Li J, Dong G, Yue J (February 2014). "The endogenous substrates of brain CYP2D". European Journal of Pharmacology. 724: 211–218. doi:10.1016/j.ejphar.2013.12.025. PMID 24374199.
- Romero-Calderón R, Uhlenbrock G, Borycz J, Simon AF, Grygoruk A, Yee SK, Shyer A, Ackerson LC, Maidment NT, Meinertzhagen IA, Hovemann BT, Krantz DE (November 2008). "A glial variant of the vesicular monoamine transporter is required to store histamine in the Drosophila visual system". PLOS Genet. 4 (11): e1000245. doi:10.1371/journal.pgen.1000245. PMC 2570955. PMID 18989452.
Unlike other monoamine neurotransmitters, the mechanism by which the brain's histamine content is regulated remains unclear. In mammals, vesicular monoamine transporters (VMATs) are expressed exclusively in neurons and mediate the storage of histamine and other monoamines.
- Broadley KJ (March 2010). "The vascular effects of trace amines and amphetamines". Pharmacol. Ther. 125 (3): 363–375. doi:10.1016/j.pharmthera.2009.11.005. PMID 19948186.
Trace amines are metabolized in the mammalian body via monoamine oxidase (MAO; EC 1.4.3.4) (Berry, 2004) (Fig. 2) ... It deaminates primary and secondary amines that are free in the neuronal cytoplasm but not those bound in storage vesicles of the sympathetic neurone ... Similarly, β-PEA would not be deaminated in the gut as it is a selective substrate for MAO-B which is not found in the gut ...
Brain levels of endogenous trace amines are several hundred-fold below those for the classical neurotransmitters noradrenaline, dopamine and serotonin but their rates of synthesis are equivalent to those of noradrenaline and dopamine and they have a very rapid turnover rate (Berry, 2004). Endogenous extracellular tissue levels of trace amines measured in the brain are in the low nanomolar range. These low concentrations arise because of their very short half-life ... - Miller GM (January 2011). "The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity". J. Neurochem. 116 (2): 164–176. doi:10.1111/j.1471-4159.2010.07109.x. PMC 3005101. PMID 21073468.
- Khan MZ, Nawaz W (October 2016). "The emerging roles of human trace amines and human trace amine-associated receptors (hTAARs) in central nervous system". Biomed. Pharmacother. 83: 439–449. doi:10.1016/j.biopha.2016.07.002. PMID 27424325.
- Lindemann L, Hoener MC (May 2005). "A renaissance in trace amines inspired by a novel GPCR family". Trends Pharmacol. Sci. 26 (5): 274–281. doi:10.1016/j.tips.2005.03.007. PMID 15860375.
In addition to the main metabolic pathway, TAs can also be converted by nonspecific N-methyltransferase (NMT) [22] and phenylethanolamine N-methyltransferase (PNMT) [23] to the corresponding secondary amines (e.g. synephrine [14], N-methylphenylethylamine and N-methyltyramine [15]), which display similar activities on TAAR1 (TA1) as their primary amine precursors...Both dopamine and 3-methoxytyramine, which do not undergo further N-methylation, are partial agonists of TAAR1 (TA1). ...
The dysregulation of TA levels has been linked to several diseases, which highlights the corresponding members of the TAAR family as potential targets for drug development. In this article, we focus on the relevance of TAs and their receptors to nervous system-related disorders, namely schizophrenia and depression; however, TAs have also been linked to other diseases such as migraine, attention deficit hyperactivity disorder, substance abuse and eating disorders [7,8,36]. Clinical studies report increased β-PEA plasma levels in patients suffering from acute schizophrenia [37] and elevated urinary excretion of β-PEA in paranoid schizophrenics [38], which supports a role of TAs in schizophrenia. As a result of these studies, β-PEA has been referred to as the body's 'endogenous amphetamine' [39] - Wainscott DB, Little SP, Yin T, Tu Y, Rocco VP, He JX, Nelson DL (January 2007). "Pharmacologic characterization of the cloned human trace amine-associated receptor1 (TAAR1) and evidence for species differences with the rat TAAR1". The Journal of Pharmacology and Experimental Therapeutics. 320 (1): 475–85. doi:10.1124/jpet.106.112532. PMID 17038507. S2CID 10829497.
- Callier S, Snapyan M, Le Crom S, Prou D, Vincent JD, Vernier P (2003). "Evolution and cell biology of dopamine receptors in vertebrates". Biology of the Cell. 95 (7): 489–502. doi:10.1016/s0248-4900(03)00089-3. PMID 14597267. S2CID 18277786.
This "evolvability" of dopamine systems has been instrumental to adapt the vertebrate species to nearly all the possible environments.
- Vincent JD, Cardinaud B, Vernier P (1998). "[Evolution of monoamine receptors and the origin of motivational and emotional systems in vertebrates]". Bulletin de l'Académie Nationale de Médecine (in French). 182 (7): 1505–14, discussion 1515–6. PMID 9916344.
These data suggest that a D1/beta receptor gene duplication was required to elaborate novel catecholamine psychomotor adaptive responses and that a noradrenergic system specifically emerged at the origin of vertebrate evolution.
- Goulty, Matthew; Botton-Amiot, Gaelle; Rosato, Ezio; Sprecher, Simon G.; Feuda, Roberto (2023-06-06). "The monoaminergic system is a bilaterian innovation". Nature Communications. 14 (1): 3284. doi:10.1038/s41467-023-39030-2. ISSN 2041-1723. PMC 10244343.
External links
- Biogenic+monoamines at the U.S. National Library of Medicine Medical Subject Headings (MeSH)