Aromatic L-amino acid decarboxylase
Aromatic L-amino acid decarboxylase (AADC or AAAD), also known as DOPA decarboxylase (DDC), tryptophan decarboxylase, and 5-hydroxytryptophan decarboxylase, is a lyase enzyme (EC 4.1.1.28), located in region 7p12.2-p12.1.
| Aromatic L amino acid decarboxylase (DOPA decarboxylase) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| EC no. | 4.1.1.28 | ||||||||
| CAS no. | 9042-64-2 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
| DOPA decarboxylase (aromatic L-amino acid decarboxylase) | |||||||
|---|---|---|---|---|---|---|---|
 Aromatic L-amino acid decarboxylase homodimer, Human | |||||||
| Identifiers | |||||||
| Symbol | DDC | ||||||
| NCBI gene | 1644 | ||||||
| HGNC | 2719 | ||||||
| OMIM | 107930 | ||||||
| RefSeq | NM_000790 | ||||||
| UniProt | P20711 | ||||||
| Other data | |||||||
| EC number | 4.1.1.28 | ||||||
| Locus | Chr. 7 p11 | ||||||
| |||||||
Mechanism
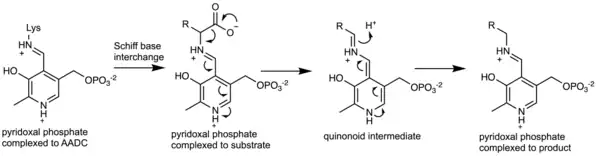
The enzyme uses pyridoxal phosphate (PLP), the active form of vitamin B6, as a cofactor. PLP is essential to the mechanism of decarboxylation in AADC. In the active enzyme, PLP is bound to lysine-303 of AADC as a Schiff base. Upon substrate binding, Lys-303 is displaced by the substrate's amine. This positions the carboxylate of the substrate within the active site such that decarboxylation is favored. Decarboxylation of the substrate produces a quinonoid intermediate, which is subsequently protonated to produce a Schiff base adduct of PLP and the decarboxylated product. Lys-303 can then regenerate the original Schiff base, releasing the product while retaining PLP.[2]
Probing this PLP-catalyzed decarboxylation, it has been discovered that there is a difference in concentration and pH dependence between substrates. DOPA is optimally decarboxylated at pH 6.7 and a PLP concentration of 0.125 mM, while the conditions for optimal 5-HTP decarboxylation were found to be pH 8.3 and 0.3 mM PLP.[3]
Structure
Aromatic L-amino acid decarboxylase is active as a homodimer. Before addition of the pyridoxal phosphate cofactor, the apoenzyme exists in an open conformation. Upon cofactor binding, a large structural transformation occurs as the subunits pull closer and close the active site. This conformational change results in the active, closed holoenzyeme.[4]
In PLP-deficient murine models, it has been observed that dopamine levels do not significantly deviate from PLP-supplemented specimens; however, the concentration of serotonin in the deficient brain model was significant. This variable effect of PLP-deficiency indicates possible isoforms of AADC with differential substrate specificity for DOPA and 5-HTP. Dialysis studies also suggest that the potential isoform responsible for DOPA decarboxylation has a greater binding affinity for PLP than that of 5-HTP decarboxylase.[3]
Regulation
AADC regulation, especially as it relates to L-DOPA decarboxylation, has been studied extensively. AADC has several conserved protein kinase A (PKA) and protein kinase G recognition sites, with residues S220, S336, S359, T320, and S429 all as potential phosphate acceptors. In vitro studies have confirmed PKA and PKG can both phosphorylate AADC, causing a significant increase in activity.[5][6] In addition, dopamine receptor antagonists have been shown to increase AADC activity in rodent models, while activation of some dopamine receptors suppresses AADC activity.[7] Such receptor mediated regulation is biphasic, with an initial short term activation followed by long term activation. The short term activation is thought to proceed through kinase activation and subsequent phosphorylation of AADC, while the sensitivity of long term activation to protein translation inhibitors suggests regulation of mRNA transcription.[8]
Reactions
AADC catalyzes several different decarboxylation reactions:[9]
- L-DOPA to dopamine – a neurotransmitter
- L-Phenylalanine to phenethylamine – a trace amine which functions as a neuromodulator
- L-Tyrosine to tyramine – a trace amine neuromodulator
- L-Histidine to histamine – a neurotransmitter
- L-Tryptophan to tryptamine – a trace amine neuromodulator
- 5-HTP to serotonin (5-hydroxytryptamine) – a neurotransmitter
However, some of these reactions do not seem to bear much or any biological significance. For example, histamine is biosynthesised strictly via the enzyme histidine decarboxylase in humans and other organisms.[10][11]
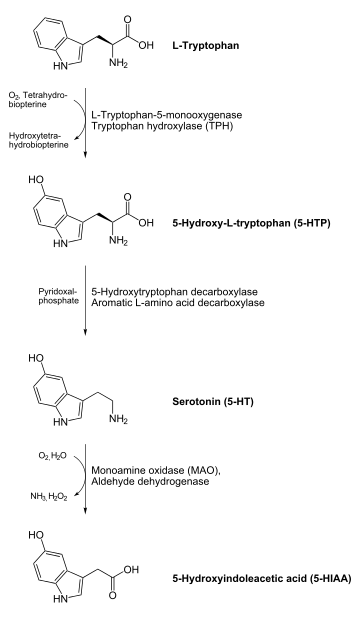
Clinical relevance
In normal dopamine and serotonin (5-HT) neurotransmitter synthesis, AADC is not the rate-limiting step in either reaction. However, AADC becomes the rate-limiting step of dopamine synthesis in patients treated with L-DOPA (such as in Parkinson's disease), and the rate-limiting step of serotonin synthesis in people treated with 5-HTP (such as in mild depression or dysthymia). AADC is inhibited by carbidopa outside of the blood brain barrier to inhibit the premature conversion of L-DOPA to dopamine in the treatment of Parkinson's.
In humans, AADC is also the rate-limiting enzyme in the formation of trace amines. Aromatic l-amino acid decarboxylase deficiency is associated with various symptoms as severe developmental delay, oculogyric crises and autonomic dysfunction. The molecular and clinical spectrum of AAAC deficiency is heterogeneous. The first case of AADC deficiency was described in twin brothers 1990. Patients can be treated with dopamine agonists, MAO inhibitors, and pyridoxine (vitamin B6).[15] Clinical phenotype and response to treatment is variable and the long-term and functional outcome is unknown. To provide a basis for improving the understanding of the epidemiology, genotype–phenotype correlation and outcome of these diseases their impact on the quality of life of patients, and for evaluating diagnostic and therapeutic strategies a patient registry was established by the noncommercial International Working Group on Neurotransmitter Related Disorders (iNTD).[16]
Immunohistochemical studies have revealed that AADC is expressed in various neuronal cell types such as serotonergic and catecholaminergic neurons. Neurons that express AADC but are not considered classical monoaminergic cell neurons are termed D cells. Cells that are immunoreactive for AADC have also been found in the human brainstem. These cells include melanin-pigmented cells that are typically designated as catecholaminergic and may also be serotonergic. Significant localization of dopaminergic cells that are also immunoreactive for AADC is reported in the substantia nigra, ventral tegmental area, and the mesencephalic reticular formation. Unlike previous reports on animal models, nonaminergic (D cells) are unlikely to be observed in the human brain.[17]
Genetics
The gene encoding the enzyme is referred to as DDC is located on chromosome 7 in humans.[18] It consists of 15 exons encoding a protein of 480 amino acids.[19] Single nucleotide polymorphisms and other gene variations have been investigated in relation to neuropsychiatric disorders, for example, a one-base pair deletion at 601 and a four-base pair deletion at 722–725 in exon 1 in relation to bipolar disorder[20] and autism. No direct correlation between gene variation and autism was found.[21]
More than 50 mutations of DDC have been correlated with AADC deficiency[22] This condition is most prevalent in Asia, presumably due to the founder effect.[23]
Alternative splicing events and promoters have been observed that lead to various forms of the AADC enzyme. Unique usage of certain promoters leads to transcription of only the first exon to produce an extra-neuronal isoform, and splicing of exon 3 leads to a product devoid of enzymatic activity. Analyses via porcine specimens have elucidated two AADC isoforms – resulting from exclusion of exon 5 and exons 5 and 6 – that lack a portion of the decarboxylating domain.[19]
See also
- Aromatic L-amino acid decarboxylase inhibitor, a class of anti-Parkinson drugs
- Aromatic amino acids
- Histidine decarboxylase
References
- PDB: 1JS3; Burkhard P, Dominici P, Borri-Voltattorni C, Jansonius JN, Malashkevich VN (November 2001). "Structural insight into Parkinson's disease treatment from drug-inhibited DOPA decarboxylase". Nature Structural Biology. 8 (11): 963–7. doi:10.1038/nsb1101-963. PMID 11685243. S2CID 19160912.
- Bertoldi M (March 2014). "Mammalian Dopa decarboxylase: structure, catalytic activity and inhibition". Archives of Biochemistry and Biophysics. 546: 1–7. doi:10.1016/j.abb.2013.12.020. PMID 24407024.
- Siow YL, Dakshinamurti K (1985). "Effect of pyridoxine deficiency on aromatic L-amino acid decarboxylase in adult rat brain". Experimental Brain Research. 59 (3): 575–81. doi:10.1007/BF00261349. PMID 3875501. S2CID 22286973.
- Giardina G, Montioli R, Gianni S, Cellini B, Paiardini A, Voltattorni CB, Cutruzzolà F (December 2011). "Open conformation of human DOPA decarboxylase reveals the mechanism of PLP addition to Group II decarboxylases". Proceedings of the National Academy of Sciences of the United States of America. 108 (51): 20514–9. Bibcode:2011PNAS..10820514G. doi:10.1073/pnas.1111456108. PMC 3251144. PMID 22143761.
- Duchemin AM, Berry MD, Neff NH, Hadjiconstantinou M (August 2000). "Phosphorylation and activation of brain aromatic L-amino acid decarboxylase by cyclic AMP-dependent protein kinase". Journal of Neurochemistry. 75 (2): 725–31. doi:10.1046/j.1471-4159.2000.0750725.x. PMID 10899948.
- Duchemin AM, Neff NH, Hadjiconstantinou M (July 2010). "Aromatic L-amino acid decarboxylase phosphorylation and activation by PKGIalpha in vitro". Journal of Neurochemistry. 114 (2): 542–52. doi:10.1111/j.1471-4159.2010.06784.x. PMID 20456015.
- Hadjiconstantinou M, Neff NH (2008). "Enhancing aromatic L-amino acid decarboxylase activity: implications for L-DOPA treatment in Parkinson's disease". CNS Neuroscience & Therapeutics. 14 (4): 340–51. doi:10.1111/j.1755-5949.2008.00058.x. PMC 6494005. PMID 19040557.
- Berry MD, Juorio AV, Li XM, Boulton AA (September 1996). "Aromatic L-amino acid decarboxylase: a neglected and misunderstood enzyme". Neurochemical Research. 21 (9): 1075–87. doi:10.1007/BF02532418. PMID 8897471. S2CID 19823573.
- "AADC". Human Metabolome database. Retrieved 17 February 2015.
- Huang H, Li Y, Liang J, Finkelman FD (2018). "Molecular Regulation of Histamine Synthesis". Frontiers in Immunology. 9: 1392. arXiv:1802.02540. doi:10.3389/fimmu.2018.01392. PMC 6019440. PMID 29973935.
- chikawa A, Tanaka S (2012). "Histamine Biosynthesis and Function". eLS. American Cancer Society. doi:10.1002/9780470015902.a0001404.pub2. ISBN 9780470015902.
- Broadley KJ (March 2010). "The vascular effects of trace amines and amphetamines". Pharmacology & Therapeutics. 125 (3): 363–375. doi:10.1016/j.pharmthera.2009.11.005. PMID 19948186.
- Lindemann L, Hoener MC (May 2005). "A renaissance in trace amines inspired by a novel GPCR family". Trends in Pharmacological Sciences. 26 (5): 274–281. doi:10.1016/j.tips.2005.03.007. PMID 15860375.
- Wang X, Li J, Dong G, Yue J (February 2014). "The endogenous substrates of brain CYP2D". European Journal of Pharmacology. 724: 211–218. doi:10.1016/j.ejphar.2013.12.025. PMID 24374199.
- Pons R, Ford B, Chiriboga CA, Clayton PT, Hinton V, Hyland K, Sharma R, De Vivo DC (April 2004). "Aromatic L-amino acid decarboxylase deficiency: clinical features, treatment, and prognosis". Neurology. 62 (7): 1058–65. doi:10.1212/WNL.62.7.1058. PMID 15079002. S2CID 12374358.
- "Patient registry".
- Kitahama K, Ikemoto K, Jouvet A, Araneda S, Nagatsu I, Raynaud B, et al. (October 2009). "Aromatic L-amino acid decarboxylase-immunoreactive structures in human midbrain, pons, and medulla". Journal of Chemical Neuroanatomy. 38 (2): 130–40. doi:10.1016/j.jchemneu.2009.06.010. PMID 19589383. S2CID 20759513.
- Scherer LJ, McPherson JD, Wasmuth JJ, Marsh JL (June 1992). "Human dopa decarboxylase: localization to human chromosome 7p11 and characterization of hepatic cDNAs". Genomics. 13 (2): 469–71. doi:10.1016/0888-7543(92)90275-W. PMID 1612608. S2CID 37950853.
- Blechingberg J, Holm IE, Johansen MG, Børglum AD, Nielsen AL (January 2010). "Aromatic l-amino acid decarboxylase expression profiling and isoform detection in the developing porcine brain". Brain Research. 1308: 1–13. doi:10.1016/j.brainres.2009.10.051. PMID 19857468. S2CID 8292103.
- Børglum AD, Bruun TG, Kjeldsen TE, Ewald H, Mors O, Kirov G, et al. (November 1999). "Two novel variants in the DOPA decarboxylase gene: association with bipolar affective disorder". Molecular Psychiatry. 4 (6): 545–51. doi:10.1038/sj.mp.4000559. PMID 10578236.
- Lauritsen MB, Børglum AD, Betancur C, Philippe A, Kruse TA, Leboyer M, Ewald H (May 2002). "Investigation of two variants in the DOPA decarboxylase gene in patients with autism". American Journal of Medical Genetics. 114 (4): 466–70. doi:10.1002/ajmg.10379. PMC 4826443. PMID 11992572.
- Wassenberg T, Molero-Luis M, Jeltsch K, Hoffmann GF, Assmann B, Blau N, et al. (January 2017). "Consensus guideline for the diagnosis and treatment of aromatic l-amino acid decarboxylase (AADC) deficiency". Orphanet Journal of Rare Diseases. 12 (1): 12. doi:10.1186/s13023-016-0522-z. PMC 5241937. PMID 28100251.
- Lee HF, Tsai CR, Chi CS, Chang TM, Lee HJ (March 2009). "Aromatic L-amino acid decarboxylase deficiency in Taiwan". European Journal of Paediatric Neurology. 13 (2): 135–40. doi:10.1016/j.ejpn.2008.03.008. PMID 18567514.
External links
- Aromatic-L-Amino-Acid+Decarboxylases at the U.S. National Library of Medicine Medical Subject Headings (MeSH)