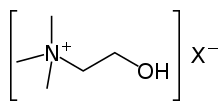
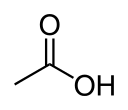
Cholinesterase
The enzyme cholinesterase (EC 3.1.1.8, choline esterase; systematic name acylcholine acylhydrolase) catalyses the hydrolysis of choline-based esters:
- an acylcholine + H2O = choline + a carboxylate
| acetylcholinesterase (Yt blood group) | |||||||
|---|---|---|---|---|---|---|---|
 | |||||||
| Identifiers | |||||||
| Symbol | ACHE | ||||||
| Alt. symbols | YT | ||||||
| NCBI gene | 43 | ||||||
| HGNC | 108 | ||||||
| OMIM | 100740 | ||||||
| RefSeq | NM_015831 | ||||||
| UniProt | P22303 | ||||||
| Other data | |||||||
| EC number | 3.1.1.7 | ||||||
| Locus | Chr. 7 q22 | ||||||
| |||||||
| butyrylcholinesterase | |||||||
|---|---|---|---|---|---|---|---|
 | |||||||
| Identifiers | |||||||
| Symbol | BCHE | ||||||
| Alt. symbols | CHE1, CHE2, E1 | ||||||
| NCBI gene | 590 | ||||||
| HGNC | 983 | ||||||
| OMIM | 177400 | ||||||
| RefSeq | NM_000055 | ||||||
| UniProt | P06276 | ||||||
| Other data | |||||||
| EC number | 3.1.1.8 | ||||||
| Locus | Chr. 3 q26.1-26.2 | ||||||
| |||||||
Several of these serve as neurotransmitters.[1] Thus, it is either of two enzymes that catalyze the hydrolysis of these cholinergic neurotransmitters, such as breaking acetylcholine into choline and acetic acid.[1] These reactions are necessary to allow a cholinergic neuron to return to its resting state after activation. For example, in muscle contraction, acetylcholine at a neuromuscular junction triggers a contraction; but for the muscle to relax afterward, rather than remaining locked in a tense state, the acetylcholine must be broken down by a choline esterase. The main type for that purpose is acetylcholinesterase (also called choline esterase I[2] or erythrocyte cholinesterase); it is found mainly in chemical synapses and red blood cell membranes. The other type is butyrylcholinesterase (also called choline esterase II[2] or plasma cholinesterase); it is found mainly in the blood plasma.
Types and nomenclature
The two types of cholinesterase are acetylcholinesterase (ACHE) and butyrylcholinesterase (BCHE). The difference between the two types has to do with their respective preferences for substrates: the former hydrolyses acetylcholine more quickly; the latter hydrolyses butyrylcholine more quickly.
The term cholinesterase is sometimes used to refer specifically to butyrylcholinesterase,[2] but this usage produces the oddity that cholinesterase and false cholinesterase (pseudocholinesterase) under that scheme mean the same thing[2] (confusingly), and acetylcholinesterase is then called true cholinesterase in contrast,[2] producing the second oddity that cholinesterase and true cholinesterase then do not mean the same thing. But such usage is now outdated; the current, unambiguous HGNC names and symbols are acetylcholinesterase (ACHE) and butyrylcholinesterase (BCHE).
Acetylcholinesterase (EC 3.1.1.7) (ACHE), also known as AChE, choline esterase I, RBC cholinesterase, or erythrocyte cholinesterase, true cholinesterase, choline esterase I, or (most formally) acetylcholine acetylhydrolase, is found primarily in the blood on red blood cell membranes, in neuromuscular junctions, and in other neural synapses. Acetylcholinesterase exists in multiple molecular forms. In the mammalian brain the majority of AChE occurs as a tetrameric, G4 form (10) with much smaller amounts of a monomeric G1 (4S) form.[3]
Butyrylcholinesterase (EC 3.1.1.8) (BCHE), also known as cholinesterase, choline esterase II, BChE, BuChE, pseudocholinesterase (PCE), plasma cholinesterase (PChE), serum cholinesterase (SChE), butylcholinesterase, or (most formally) acylcholine acylhydrolase, is produced in the liver and found primarily in blood plasma. The butyl and butyryl syllables both refer to butane with one of its terminal methyl groups substituted.
The half-life of BCHE is approximately 10 to 14 days.[4] BCHE levels may be reduced in patients with advanced liver disease. The decrease must be greater than 75% before significant prolongation of neuromuscular blockade occurs with succinylcholine.[5][6]
Discovery
In 1968, Walo Leuzinger et al. successfully purified and crystallized acetylcholinesterase from electric eels at Columbia University, New York.[7][8]
The 3D structure of acetylcholinesterase was first determined in 1991 by Joel Sussman et al. using protein from the Pacific electric ray.[9]
Clinically useful quantities of butyrylcholinesterase were synthesized in 2007 by PharmAthene, through the use of genetically modified goats.[10]
Clinical significance
An absence or mutation of the BCHE enzyme leads to a medical condition known as pseudocholinesterase deficiency. This is a silent condition that manifests itself only when people that have the deficiency receive the muscle relaxants succinylcholine or mivacurium during a surgery.
Pseudocholinesterase deficiency may also affect local anaesthetic selection in dental procedures. The enzyme plays an important role in the metabolism of ester-based local anaesthetics, a deficiency lowers the margin of safety and increases the risk of systemic effects with this type of anaesthetic. The selection of an amide-based solution is recommended in such patients.
Elevation of plasma BCHE levels was observed in 90.5% of cases of acute myocardial infarction.[11]
The presence of ACHE in the amniotic fluid may be tested in early pregnancy. A sample of amniotic fluid is removed by amniocentesis, and presence of ACHE can confirm several common types of birth defect, including abdominal wall defects and neural tube defects.[12]
BCHE can be used as a prophylactic agent against nerve gas and other organophosphate poisoning.[10]
Some early research points to genetic butylcholinesterase deficiency as a possible candidate component in sudden infant death syndrome.[13]
The enzyme Acetylcholin esterase, and its inhibition, plays a role in the development of myofascial trigger points and the associated myofascial pain syndrome. By injecting a mouse with acetylcholin esterase inhibitors and electrical stimulation, the muscle develops trigger points.[14][15]
Inhibitors
A cholinesterase inhibitor (or "anticholinesterase") suppresses the action of the enzyme. Because of its essential function, chemicals that interfere with the action of cholinesterase are potent neurotoxins, causing excessive salivation and eye-watering in low doses, followed by muscle spasms and ultimately death (examples are some snake venoms, and the nerve gases sarin and VX). One counteracting medication is pralidoxime. The so-called nerve gases and many substances used in insecticides have been shown to act by combining with a residue of serine in the active site of acetylcholine esterase, inhibiting the enzyme completely. The enzyme acetylcholine esterase breaks down the neurotransmitter acetylcholine, which is released at nerve and muscle junctions, in order to allow the muscle or organ to relax. The result of acetylcholine esterase inhibition is that acetylcholine builds up and continues to act so that any nerve impulses are continually transmitted and muscle contractions do not stop.
Among the most common acetylcholinesterase inhibitors are phosphorus-based compounds, which are designed to bind to the active site of the enzyme. The structural requirements are a phosphorus atom bearing two lipophilic groups, a leaving group (such as a halide or thiocyanate), and a terminal oxygen. The entry on Lawesson's reagent has some details on one sub-class of the phosphorus-based compounds.
Some benzodiazepines, e.g. temazepam have an inhibitory effect on cholinesterase.[16]
Cholinesterase levels can be used as an indirect marker of arsenic exposure.[17]
Outside of biochemical warfare, anticholinesterases are also used for reversing medication induced paralysis during anesthesia; as well as in the treatment of myasthenia gravis, glaucoma, and Alzheimer's disease. Such compounds are used for killing insects in a range of products including sheep dip, organophosphate pesticides, and carbamate pesticides. In addition to acute poisoning as described above, a semi-acute poisoning characterized by strong mental disturbances can occur. Also, prolonged exposure can cause birth defects.
Additional images
References
- Colović MB, Krstić DZ, Lazarević-Pašti TD, Bondžić AM, Vasić VM (May 2013). "Acetylcholinesterase inhibitors: pharmacology and toxicology". Current Neuropharmacology. Bentham Science Publishers Ltd. 11 (3): 315–35. doi:10.2174/1570159x11311030006. PMC 3648782. PMID 24179466.
- Elsevier, Dorland's Illustrated Medical Dictionary, Elsevier, archived from the original on 2014-01-11, retrieved 2015-09-24.
- Wang R, Tang XC (2005). "Neuroprotective effects of huperzine A. A natural cholinesterase inhibitor for the treatment of Alzheimer's disease". Neuro-Signals. 14 (1–2): 71–82. doi:10.1159/000085387. PMID 15956816.
- Whittaker M (1980). "Plasma cholinesterase variants and the anaesthetist". Anaesthesia. 35 (2): 174–197. doi:10.1111/j.1365-2044.1980.tb03800.x. PMID 6992635. S2CID 32806785.
- Barash PG, Cullen BF, Stoelting RK (2006). Handbook of Clinical Anesthesia (5th ed.). Philadelphia: Lippincott Williams & Wilkins. pp. 546–9. ISBN 978-0-7817-5745-4.
- Miller RD (2005). Miller's Anesthesia (6th ed.). Philadelphia, Penns.: Elsevier/Churchill Livingstone. pp. 487–8. ISBN 978-0-443-06618-4.
- Leuzinger W, Baker AL (Feb 1967). "Acetylcholinesterase, I. Large-scale purification, homogeneity, and amino Acid analysis". Proceedings of the National Academy of Sciences of the United States of America. 57 (2): 446–51. Bibcode:1967PNAS...57..446L. doi:10.1073/pnas.57.2.446. PMC 335526. PMID 16591490.
- Leuzinger W, Baker AL, Cauvin E (Feb 1968). "Acetylcholinesterase. II. Crystallization, absorption spectra, isoionic point". Proceedings of the National Academy of Sciences of the United States of America. 59 (2): 620–3. Bibcode:1968PNAS...59..620L. doi:10.1073/pnas.59.2.620. PMC 224717. PMID 5238989.
- Sussman JL, Harel M, Frolow F, Oefner C, Goldman A, Toker L, Silman I (Aug 1991). "Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein". Science. 253 (5022): 872–9. Bibcode:1991Sci...253..872S. doi:10.1126/science.1678899. PMID 1678899. S2CID 28833513.
- Huang YJ, Huang Y, Baldassarre H, Wang B, Lazaris A, Leduc M, Bilodeau AS, Bellemare A, Côté M, Herskovits P, Touati M, Turcotte C, Valeanu L, Lemée N, Wilgus H, Bégin I, Bhatia B, Rao K, Neveu N, Brochu E, Pierson J, Hockley DK, Cerasoli DM, Lenz DE, Karatzas CN, Langermann S (Aug 2007). "Recombinant human butyrylcholinesterase from milk of transgenic animals to protect against organophosphate poisoning". Proceedings of the National Academy of Sciences of the United States of America. 104 (34): 13603–8. Bibcode:2007PNAS..10413603H. doi:10.1073/pnas.0702756104. PMC 1934339. PMID 17660298.
- "Nerve gas antidote made by goats". BBC News. 24 July 2007.
- Shinde R, Chatterjea MN (2005). Textbook of Medical Biochemistry (6th ed.). New Delhi: Jaypee Brothers Medical Publications (P) Ltd. p. 565. ISBN 978-9350254844.
- FBR Resource Guide: Acetylcholinesterase-Amniotic Fluid Archived 2007-06-25 at the Wayback Machine. Foundation for Blood Research (September 7, 2007). Retrieved on 2007-11-21.
- Harrington, C. T.; Hafid, N. A.; Waters, K. A. (2022-05-06). "Butyrylcholinesterase is a potential biomarker for Sudden Infant Death Syndrome". eBioMedicine. 80. doi:10.1016/j.ebiom.2022.104041. PMC 9092508. PMID 35533499.
- Mense, S.; Simons, D.G.; Hoheisel, U.; Quenzer, B. (2003). "Lesions of rat skeletal muscle after local block of acetylcholinesterase and neuromuscular stimulation". J Appl Physiol. 94 (6): 2494–2901. doi:10.1152/japplphysiol.00727.2002. PMID 12576409. S2CID 1829156. Retrieved 2023-09-24.
- Simons, David G. (February 2004). "Review of enigmatic MTrPs as a common cause of enigmatic musculoskeletal pain and dysfunction". Journal of Electromyography and Kinesiology. 14 (1): 95–107. doi:10.1016/j.jelekin.2003.09.018. ISSN 1050-6411. PMID 14759755.
- Holmes JH, Kanfer I, Zwarenstein H (Aug 1978). "Effect of benzodiazepine derivatives on human blood cholinesterase in vitro". Research Communications in Chemical Pathology and Pharmacology. 21 (2): 367–70. PMID 29327.
- Ali N, Hoque MA, Haque A, Salam KA, Karim MR, Rahman A, Islam K, Saud ZA, Khalek MA, Akhand AA, Hossain M, Mandal A, Karim MR, Miyataka H, Himeno S, Hossain K (2010). "Association between arsenic exposure and plasma cholinesterase activity: a population based study in Bangladesh". Environmental Health. 9: 36. doi:10.1186/1476-069X-9-36. PMC 2911418. PMID 20618979.
External links
- ATSDR Case Studies in Environmental Medicine: Cholinesterase Inhibitors, Including Insecticides and Chemical Warfare Nerve Agents U.S. Department of Health and Human Services
- Movies at weizmann.ac.il showing the structure of acetylcholinesterase and interactions with various inhibitors.
- Proteopedia Acetylcholinesterase
- PDB Molecule of the Month Cholinesterase
- Acetylcholinesterase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Pseudocholinesterase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)