Malouetine
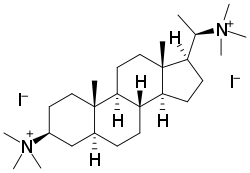
Malouetine is an aminosteroid neuromuscular blocking agent and antinicotinic alkaloid isolated from Malouetia spp.[1]
 | |
| Names | |
|---|---|
| IUPAC name
[(3S,5S,8R,9S,10S,13S,14S)-10,13-Dimethyl-17-[(1S)-1-(trimethylazaniumyl)ethyl]-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl]-trimethylazanium diiodide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C27H52I2N2 | |
| Molar mass | 658.536 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
The structure of malouetine inspired the development of modern aminosteroid muscle relaxants such as pancuronium bromide and vecuronium bromide by workers at Organon.[2][3][4]
References
- Janot MM; Laine F; Goutarel R (1960). "Steroid alkaloids. V. Alkaloids of Malouetia bequaertiana e. Woodson (Apocynaceae): Funtuphyllamine B and malouetine. Preliminary communication". Annales Pharmaceutiques Françaises. 18: 673–677. PMID 13789457.
- Alauddin, M.; Caddy, B.; Lewis, J.J.; Martin-Smith, M.; Sugrue, M.F. (January 1965). "Non-depolarising neuromuscular blockade by 3α,17α-bis(quaternary ammonium) 5α-androstanes". Journal of Pharmacy and Pharmacology. 17 (1): 55–59. doi:10.1111/j.2042-7158.1965.tb07569.x.
- Buckett, W.R.; Hewett, C.L.; Savage, D.S. (October 1973). "Pancuronium bromide and other steroidal neuromuscular blocking agents containing acetylcholine fragments". Journal of Medicinal Chemistry. 16 (10): 1116–24. doi:10.1021/jm00268a011. PMID 4356139.
- McKenzie, A.G. (June 2000). "Prelude to pancuronium and vecuronium". Anaesthesia. 55 (6): 551–556. doi:10.1046/j.1365-2044.2000.01423.x.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.