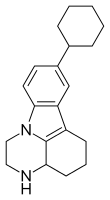
Tetrindole
Tetrindole was a drug candidate that functions by reversibly inhibiting monoamine oxidase A; it was first synthesized in Moscow in the early 1990s.[1] Tetrindole is similar in its chemical structure to pirlindole (Pyrazidol), and metralindole.[2]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| PubChem CID | |
| ChemSpider | |
| ChEBI | |
| Chemical and physical data | |
| Formula | C20H26N2 |
| Molar mass | 294.442 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
References
- Medvedev AE, Kirkel AA, Kamyshanskaya NS, Moskvitina TA, Axenova LN, Gorkin VZ, et al. (January 1994). "Monoamine oxidase inhibition by novel antidepressant tetrindole". Biochemical Pharmacology. 47 (2): 303–8. doi:10.1016/0006-2952(94)90021-3. PMID 8304974.
- Ramsay RR, Gravestock MB (March 2003). "Monoamine oxidases: to inhibit or not to inhibit". Mini Reviews in Medicinal Chemistry. 3 (2): 129–36. doi:10.2174/1389557033405287. PMID 12570845.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.