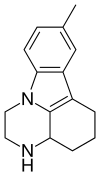
Pirlindole
Pirlindole (Lifril, Pyrazidol) is mainly a reversible inhibitor of monoamine oxidase A (RIMA) and secondly a SNRI which was developed and is used in Russia as an antidepressant.[2] It is structurally and pharmacologically related to metralindole.
 | |
| Clinical data | |
|---|---|
| Trade names | Pirazidol |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 20–30% |
| Protein binding | 95% |
| Metabolism | hepatic |
| Onset of action | 2 to 8 hours |
| Elimination half-life | up to 8 days [1] |
| Excretion | urine (50–70%), feces (25–45%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C15H18N2 |
| Molar mass | 226.323 g·mol−1 |
| 3D model (JSmol) | |
| |
Synthesis
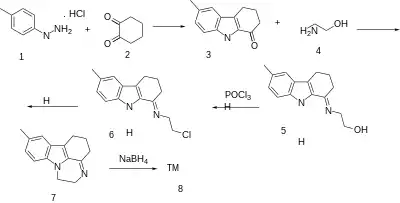
The Fischer indole synthesis between p-Tolylhydrazine Hydrochloride [637-60-5] (1) and 1,2-Cyclohexanedione [765-87-7] (2) gives 6-methyl-2,3,4,9-tetrahydrocarbazol-1-one [3449-48-7] (3). Imine formation with ethanolamine [141-43-5] (4) gives CID:2838578 (5). Halogenation with phosphorus oxychloride gives (6).[13] Intramolcular alkylation with the indole nitrogen resulted in Dehydropirlindole [75804-32-9] (7). Reduction of the imine with sodium borohydride completes the synthesis of pirlindole (8).
See also
References
- Pöldinger W (1985). "Pirlindole: results of an open clinical study in out-patients and of a double-blind study against maprotiline.". Psychiatry the State of the Art. Boston, MA.: Springer. pp. 283–289. doi:10.1007/978-1-4613-2363-1_44. ISBN 978-1-4613-2363-1.
- Bruhwyler J, Liégeois JF, Géczy J (July 1997). "Pirlindole: a selective reversible inhibitor of monoamine oxidase A. A review of its preclinical properties". Pharmacological Research. 36 (1): 23–33. doi:10.1006/phrs.1997.0196. PMID 9368911.
- Filitis LN, Fedotova OA, Akalaeva TV, Bokanov AI, Ivanov PY, Neustroeva VD, Nyrkova VG, Pershin GN, Shvedov VI (1986). "Tetrahydrocarbazole derivatives and their antitubercular activity in vitro. I. N-Substituted hexahydro-1H-pyrazino[3,2,1-j,k]carbazoles". Khimiko-Farmatsevticheskii Zhurnal (in Russian). 20 (3): 300–303.
- Ivanov PY, Alekseeva LM, Bokanov AI, Shvedov VI, Sheinker YN (January 1987). "New approach to the synthesis of pyrazidol". Pharmaceutical Chemistry Journal. 21 (1): 62–65. doi:10.1007/BF00764890..
- De Tullio P, Felikidis A, Pirotte B, Liégeois JF, Stachow M, Delarge J, Ceccato A, Hubert P, Crommen J, Géczy J (1998). "First Preparative Enantiomer Resolution of Pirlindole, a Potent Antidepressant Drug". Helvetica Chimica Acta. 81 (3–4): 539–547. doi:10.1002/hlca.19980810307. ISSN 0018-019X..
- , FR 2132514 (1972 to Inst Im Sergo); CA, 78, 124628r
- Massimo Ferrari, et al. EP 1044976 (2002 to Erregierre SpA).
- Massimo Ferrari, et al. CZ20001348 (2000).
- DE 2114230
- GB 1340529
- Chen Weidong, et al. CN 110950873 (2020 to Henan University).
- Carla Patricia Da Costa Pereira Rosa, et al. WO 2018193415 (to Tecnimede Sociedade Tecnico Medicinal SA).
- "N-(2-chloroethyl)-6-methyl-2,3,4,9-tetrahydrocarbazol-1-imine". PubMed. U.S. National Library of Medicine.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.