Alpidem
Alpidem, sold under the brand name Ananxyl, is a nonbenzodiazepine anxiolytic medication which was briefly used to treat anxiety disorders but is no longer marketed.[3] It was previously marketed in France, but was discontinued due to liver toxicity.[3] Alpidem is taken by mouth.[1]
 | |
| Clinical data | |
|---|---|
| Trade names | Ananxyl |
| Other names | SL 80.0342; SL800342; SL-800342 |
| Routes of administration | Oral administration |
| Drug class | Nonbenzodiazepine; GABAA receptor positive allosteric modulator; Anxiolytic |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 32–35% (estimated)[1][2] |
| Protein binding | 99.4%[1] |
| Metabolism | Extensive (hydroxylation, dealkylation, conjugation)[1] |
| Metabolites | Many (some active)[1] |
| Onset of action | 1.0–2.5 hours (Cmax)[1] |
| Elimination half-life | Young adults: 19 hours (7–44 hours)[1] Elderly: 22.6 ± 2.3 hours[1] Children: 11.4 ± 1.9 hours[1] |
| Excretion | Mainly feces[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.216.305 |
| Chemical and physical data | |
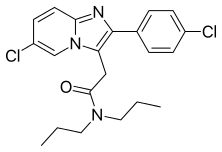
| Formula | C21H23Cl2N3O |
| Molar mass | 404.34 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Side effects of alpidem include sedation, fatigue, dizziness, and headache, among others.[3][2][4] It has much less to no impact on cognition, memory, and psychomotor function relative to benzodiazepines.[3][5] Similarly, no rebound anxiety or withdrawal symptoms have been observed with alpidem.[3][2] Rarely, alpidem can cause serious liver toxicity, including liver failure and death.[3] Alpidem is a nonbenzodiazepine of the imidazopyridine family, structurally related to the Z-drug zolpidem,[1] and acts as a GABAA receptor positive allosteric modulator of the benzodiazepine site of the receptor complex.[3] In contrast to zolpidem however, alpidem has anxiolytic effects rather than sedative or hypnotic effects at normal therapeutic doses.[3]
Alpidem was first described by 1982[6][7] and was introduced for medical use in France in 1991.[3][8][9] It was also under development for use in other countries in the 1990s, but development was discontinued and the drug was never marketed in any other country.[8][9] Alpidem was withdrawn from the market in France in 1993 due to liver toxicity.[10][11][12][13][3]
Medical uses
Alpidem was approved for the treatment of generalized anxiety disorder and possibly also other anxiety problems.[3][14][8] By 1990, 17 clinical studies including more than 1,500 patients had been conducted in Europe studying alpidem for the treatment of anxiety.[2][3] In clinical trials, alpidem demonstrated effectiveness in the treatment of chronic and situational anxiety, including stress-related anxiety, generalized anxiety, and adjustment disorder (situational depression) with anxiety.[2][14] It also showed preliminary effectiveness in institutionalized individuals with chronic psychosis and high anxiety levels.[2][15] The effectiveness of alpidem for panic disorder, on the other hand, is understudied and uncertain.[14][16]
The anxiolytic effects of alpidem are described as rapid, robust, and maintained in the long-term.[3][2] For situational anxiety, the anxiolytic effects of alpidem onset within 1.5 to 2 hours, whereas for chronic anxiety disorders the effects onset within 3 to 5 days in most cases.[2] No indications of tolerance to its anxiolytic effects or need for dose increases have been observed.[2] In people with anxiety taking alpidem, improvement in mood and sleep have also been found.[4]
The anxiolytic effectiveness of alpidem, for example measured by reductions on the Hamilton Anxiety Rating Scale (HAM-A), was superior to placebo and comparable or equivalent to that of benzodiazepines including diazepam (10–15 mg/day), lorazepam (1–6 mg/day), and clorazepate (30 mg/day) in directly comparative randomized controlled trials.[3][2][4][14] Alpidem has also been directly compared with buspirone (20–30 mg/day) for generalized anxiety disorder.[17] Relative to buspirone, it was found to produce more rapid improvement, to have significantly greater effectiveness, and to have fewer side effects and a lower discontinuation rate.[17]
The recommended dose of alpidem was 75 to 150 mg total per day, given in single doses of 25 to 75 mg two to three times per day.[3][18][19]
Side effects
Alpidem is described as well-tolerated.[2] Side effects include sedation (6–8%; dose-dependent), fatigue (3–4%), dizziness (3–4%), and headache (2–3%), among others.[3] It is reported to have minimal sedative effects and to have virtually no negative effects on cognition, memory, and psychomotor function at therapeutic doses.[3][2][4] However, some impairment of vigilance and psychomotor function has been reported at high doses (100–200 mg).[3][2] In addition, driving ability has been studied with alpidem and has been found to be impaired.[21][22] The central side effects of alpidem were found to be no worse in elderly people than in young adults.[3][23]
Alpidem does not alter sleep architecture as measured by electroencephalography.[2] In laboratory tests, 0.9% of patients treated with alpidem showed alterations.[2] No adverse effects on cardiovascular or respiratory function were seen in clinical trials.[2]
No rebound anxiety or withdrawal symptoms have been observed with alpidem after abrupt discontinuation following 4 weeks to 6–12 months of treatment.[3][2][4] Conversely, substantial withdrawal symptoms, including rebound anxiety, were observed with lorazepam.[4]
The side effects of alpidem are described as quite different from those of benzodiazepines.[3] In directly comparative trials, alpidem produced similar anxiolytic effects with less fatigue, asthenia, depressive mood, and psychomotor impairment than benzodiazepines, while rates of somnolence and drowsiness were comparable to benzodiazepines but described as milder in severity.[2][4] Whereas benzodiazepines commonly produce dizziness, muscle weakness, fatigue, and sleepiness as side effects, these are not prominent adverse effects with doses of alpidem that have similar anxiolytic effectiveness.[3] The lack of withdrawal or rebound symptoms with alpidem upon discontinuation is also in contrast to benzodiazepines.[2] In addition, alpidem significantly antagonized the amnestic effects of lorazepam and showed similar trends for other cognitive measures in a clinical study in which the two drugs were combined and assessed for interaction.[3][24]
Following marketing authorization in France, several cases of severe liver toxicity were reported in people taking alpidem.[3][25] This resulted in one death and several cases of liver transplantation.[3][25][26] As a result, alpidem was soon withdrawn from the market.[3] The liver toxicity of alpidem was subsequently characterized in preclinical research.[27][28][29][19] It may be related to interactions of alpidem with the translocator protein (TSPO), which is present in high amounts in the liver and which may mediate toxic effects in this tissue.[28][29][17][19]
Overdose
Little information is available on overdose with alpidem.[2] Doses of as high as 300 mg/day, which is 2 to 4 times the recommended total daily dose, were assessed in clinical trials.[2][3][30]
Interactions
Alpidem may interact with alcohol, but to a lesser extent than benzodiazepines.[4]
Pharmacology
Pharmacodynamics
Alpidem is a GABAA receptor positive allosteric modulator (GABAkine),[31] specifically acting as an agonist of the benzodiazepine site of the receptor complex (formerly known as the central benzodiazepine receptor (CBR)).[3] In addition to its affinity for the benzodiazepine site of the GABAA receptor (Ki = 1–28 nM), alpidem has similarly high affinity for the translocator protein (TSPO) (formerly the peripheral benzodiazepine receptor (PBR)) (Ki = 0.5–7 nM).[32][33][34][35][36] Alpidem shows more than 500-fold selectivity for α1 subunit-containing GABAA receptors over α5 subunit-containing GABAA receptors[3] and 80-fold selectivity for α1 subunit-containing GABAA receptors over α3 subunit-containing GABAA receptors.[37] However, alpidem has also been described as potently modulating α1, α2, and α3 subunit-containing GABAA receptors with no effect on α5 subunit-containing GABAA receptors.[38] Findings appear to be mixed on whether alpidem is a partial agonist or a full agonist of the benzodiazepine site of the GABAA receptor.[3] In animals, alpidem has anxiolytic-like effects in some but not all models, weak anticonvulsant effects, and weak or no sedative, amnesic, ataxic, or muscle relaxant effects.[3][5][39][30] High doses of alpidem antagonize the sedative and muscle relaxant effects of diazepam in animals.[39] Flumazenil has been shown to antagonize the anxiolytic and anticonvulsant effects of alpidem in animals.[5] Besides acting directly via the GABAA receptor, interactions with the TSPO might also contribute to the anxiolytic effects of alpidem.[40][32][41][38] This protein mediates promotion of neurosteroidogenesis in the brain, for instance of allopregnanolone.[40][32][41]
Alpidem is structurally related to zolpidem, and both alpidem and zolpidem are GABAA receptor positive allosteric modulators of the benzodiazepine site with preference for α1 subunit-containing receptors.[3][14] Both alpidem and zolpidem have very low affinity for α5 subunit-containing GABAA receptors, in contrast to benzodiazepines.[42][43] Similarly, both alpidem and zolpidem are selective for γ2 subunit-containing GABAA receptors, with very low affinity for γ1 and γ3 subunit-containing GABAA receptors, in contrast to other Z-drugs and to diazepam.[44] Alpidem has very high affinity for the TSPO, while zolpidem has very low affinity for this protein.[14][45][46] The affinity of alpidem for the TSPO (also previously known as the ω3 receptor)[47] was once the highest of any drug known.[45] Although benzodiazepines like diazepam are also known to bind to the TSPO, the affinity of alpidem for this protein is at least 3,000-fold higher in comparison.[45] Whereas zolpidem shows hypnotic and sedative effects and is used to treat insomnia, alpidem shows mainly anxiolytic effects and is used to treat anxiety disorders.[3][14] Alpidem was developed before the widespread use of recombinant GABAA receptors.[3] Hence, its pharmacological profile at the GABAA receptors, including its profile at different subpopulations of these receptors, has never been fully characterized.[3]
The pharmacodynamic mechanisms underlying the anxioselective (anxiolytic-selective) profile of alpidem as a GABAA receptor positive allosteric modulator are unclear.[3][48] In any case, subtype selectivity for different populations of GABAA receptors, partial agonism of the benzodiazepine site of the GABAA receptor, and/or interactions with the TSPO may potentially all be involved.[3][45][5][49][39][50][51] Although anxioselective profiles have been observed for many GABAA receptor positive allosteric modulators in preclinical research, alpidem is the only GABAA receptor positive allosteric modulator for which anxioselective effects have been unambiguously demonstrated in human clinical trials.[3] Ocinaplon has also shown preliminary signs of an anxioselective profile in clinical studies, but development of this agent was discontinued in late-stage trials due to findings of elevated liver enzymes in a small subset of patients.[3] GABAA receptor positive allosteric modulators with selectivity for α2 and α3 subunit-containing GABAA receptors over α1 subunit-containing GABAA receptors, for instance adipiplon, L-838,417, and darigabat—among others, have been and are under investigation as potential anxioselective agents.[3][31][52] However, no such drugs have yet completed clinical development or been marketed for medical use.[31][52][48][38] Despite many developmental failures, alpidem serves as a potential proof of concept that anxioselective GABAA receptor positive allosteric modulators may be possible.[3][48][38] However, if interactions with the TSPO are key to the anxiolytic effects of alpidem, then this may not actually be the case.[41]
Absorption
Alpidem is taken via oral administration.[1] The absorption of alpidem is rapid and it reaches peak levels after 1.0 to 2.5 hours.[1] Its overall bioavailability is estimated to be approximately 32 to 35%, but no precise value for absolute bioavailability has been determined.[1][2] Absorption of alpidem as indicated by peak and area-under-the-curve levels is linear across a dose range of 25 to 100 mg.[1] Food increases the bioavailability of alpidem by 15 to 20%.[1]
Distribution
Alpidem is a highly lipophilic compound and in animals is extensively distributed into lipid-rich tissues.[1] Similarly, alpidem has been shown to cross the blood–brain barrier in animals, and showed a brain/plasma ratio of about 2.0 to 2.5 following systemic administration.[1] This is related to significantly slower efflux of alpidem from the brain than entry.[1] The active metabolites of alpidem are also brain-penetrant, although occur in the brain at levels lower than those of alpidem.[1] Alpidem may be concentrated more in lipid-rich white matter brain structures than grey matter structures.[1] In humans, the volume of distribution of alpidem is large at 8.7 L•kg−1.[1] The plasma protein binding of alpidem is 99.4%, with similar isolated fractions bound to albumin (97.0%) and α1-acid glycoprotein (97.3%).[1] The free fraction of alpidem is slightly higher in people with cirrhosis (0.86 ± 0.06%) and renal failure (0.72 ± 0.03%) relative to normal individuals (0.61 ± 0.05%).[1]
Metabolism
Alpidem is extensively metabolized, including by hydroxylation, dealkylation, and conjugation.[1] Many metabolites of alpidem have been identified, and some of these metabolites may contribute to its pharmacological activity.[1]
Elimination
Alpidem is eliminated mainly in feces, with less than 0.1% excreted in urine.[1] A majority of alpidem is eliminated within 48 to 72 hours following oral dosing.[1] Only trace amounts of unchanged alpidem are found in feces and urine.[1] The metabolites of alpidem are excreted mainly in via the bile in feces, with less than 5% eliminated via urine.[1]
The elimination half-life of alpidem was mean 18.8 ± 0.8 hours (range 7 to 44 hours) following a single 50-mg oral dose given to young individuals.[1] In elderly individuals, a trend toward a longer half-life was observed (22.6 ± 2.3 hours).[1] Conversely, in children age 8 to 12 years, the half-life of alpidem was considerably reduced (11.4 ± 1.9 hours).[1] The half-lives of alpidem and its metabolites are significantly prolonged in people with hepatic impairment.[1] Conversely, the half-lives of alpidem and its metabolites were unchanged in people with different stages of renal impairment, though plasma concentrations were significantly increased.[1] The clearance of alpidem was estimated to be 0.86 ± 0.04 L•h−1•kg−1 in healthy individuals.[1]
Chemistry
Alpidem is a nonbenzodiazepine, and hence is not structurally related to benzodiazepines.[1][53] It is a member of the imidazopyridine group of compounds.[1][14] Alpidem is structurally related to the Z-drug zolpidem, which is also an imidazopyridine.[1][14]
History
Alpidem was developed by Synthélabo Recherche (subsequently Sanofi-Synthélabo and now Sanofi-Aventis).[4][9] It was developed under the code name SL 80.0342 and was first described in the literature by 1982.[54][6][7][8] Alpidem was introduced for medical use in France in 1991.[3][8][9] It was also undergoing development in the 1990s for use in other countries such as the United States and other European countries like Germany, the Netherlands, and Spain.[8] The drug reached phase 3 clinical trials in these countries.[8] However, development in the United States was halted in 1992 due to "divergent results".[55][8] All development in other countries was discontinued by 1999.[8] Alpidem was withdrawn from the market in France in 1993 due to liver toxicity.[10][11][12][13][3] It was never marketed in any other country.[8][54][9]
Society and culture
Names
Alpidem is the generic name of the drug and its INNTooltip International Nonproprietary Name, USANTooltip United States Adopted Name, BANTooltip British Approved Name, and DCFTooltip Dénomination Commune Française.[6][54] The developmental code name of alpidem was SL 80.0342.[54][8] Alpidem was previously marketed under the brand name Ananxyl.[6][54]
See also
References
- Durand A, Thénot JP, Bianchetti G, Morselli PL (1992). "Comparative pharmacokinetic profile of two imidazopyridine drugs: zolpidem and alpidem". Drug Metabolism Reviews. 24 (2): 239–266. doi:10.3109/03602539208996294. PMID 1576937.
- Morselli PL (May 1990). "On the therapeutic action of alpidem in anxiety disorders: an overview of the European data". Pharmacopsychiatry. 23 (Suppl 3): 129–134. doi:10.1055/s-2007-1014549. PMID 1974073.
- Skolnick P (November 2012). "Anxioselective anxiolytics: on a quest for the Holy Grail". Trends in Pharmacological Sciences. 33 (11): 611–620. doi:10.1016/j.tips.2012.08.003. PMC 3482271. PMID 22981367.
- Morton S, Lader M (May 1990). "Studies with alpidem in normal volunteers and anxious patients". Pharmacopsychiatry. 23 (Suppl 3): 120–123. doi:10.1055/s-2007-1014547. PMID 1974071.
- Zivkovic B, Morel E, Joly D, Perrault G, Sanger DJ, Lloyd KG (May 1990). "Pharmacological and behavioral profile of alpidem as an anxiolytic". Pharmacopsychiatry. 23 (Suppl 3): 108–113. doi:10.1055/s-2007-1014545. PMID 1974069.
- J. Elks, ed. (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 33–. ISBN 978-1-4757-2085-3. OCLC 1058412474.
- Saletu B, Grünberger J, Linzmayer L (April 1986). "Pharmacokinetic and dynamic studies with a new anxiolytic imidazo-pyridine alpidem utilizing pharmaco-EEG and psychometry". International Clinical Psychopharmacology. 1 (2): 145–164. doi:10.1097/00004850-198604000-00006. PMID 2883214.
- "Alpidem - Sanofi-Synthelabo - AdisInsight".
- "Micromedex Products: Please Login".
- Guengerich FP (2011). "Mechanisms of drug toxicity and relevance to pharmaceutical development". Drug Metabolism and Pharmacokinetics. 26 (1): 3–14. doi:10.2133/dmpk.dmpk-10-rv-062. PMC 4707670. PMID 20978361.
- "ANANXYL WITHDRAWN FROM MARKET IN FRANCE - Pharmaceutical industry news".
- "La commercialisation de l'Ananxyl est suspendue pour un an". Le Monde.fr. 26 October 1993.
- WHO Drug Information Vol. 8, No. 2, 1994, page 64
- Kunovac JL, Stahl SM (December 1995). "Future directions in anxiolytic pharmacotherapy". The Psychiatric Clinics of North America. 18 (4): 895–909. doi:10.1016/S0193-953X(18)30030-3. PMID 8748388.
- Kishi T, Inada K, Matsui Y, Iwata N (October 2017). "Z-drug for schizophrenia: A systematic review and meta-analysis". Psychiatry Research. 256: 365–370. doi:10.1016/j.psychres.2017.06.063. PMID 28686934. S2CID 13656676.
- Schneier FR, Carrasco JL, Hollander E, Campeas R, Fallon B, Saoud JB, et al. (April 1993). "Alpidem in the treatment of panic disorder". Journal of Clinical Psychopharmacology. 13 (2): 150–153. doi:10.1097/00004714-199304000-00011. PMID 8096527.
- Potokar J, Nutt DJ (April 1994). "Anxiolytic Potential of Benzodiazepine Receptor Partial Agonists". CNS Drugs. 1 (4): 305–315. doi:10.2165/00023210-199401040-00007. ISSN 1172-7047. S2CID 71162271.
- Joris C. Verster; S. R. Pandi-Perumal; Jan G. Ramaekers; Johan J. de Gier, eds. (29 August 2009). Drugs, Driving and Traffic Safety. Springer Science & Business Media. pp. 301–. ISBN 978-3-7643-9923-8. OCLC 1005763473.
- "NCATS Inxight Drugs — ALPIDEM".
- Mokrov GV, Deeva OA, Gudasheva TA (2021). "The Ligands of Translocator Protein: Design and Biological Properties". Current Pharmaceutical Design. 27 (2): 217–237. doi:10.2174/1381612826666200903122025. PMID 32881658. S2CID 221498255.
- Verster J, Veldhuijzen D, Volkerts E (1 June 2005). "Is it Safe to Drive a Car when Treated with Anxiolytics? Evidence from on-the-Road Driving Studies During Normal Traffic". Current Psychiatry Reviews. 1 (2): 215–225. doi:10.2174/1573400054065613. ISSN 1573-4005.
- Griffin CE, Kaye AM, Bueno FR, Kaye AD (2013). "Benzodiazepine pharmacology and central nervous system-mediated effects". The Ochsner Journal. 13 (2): 214–223. PMC 3684331. PMID 23789008.
- Hindmarch I (May 1990). "Alpidem and psychological performance in elderly subjects". Pharmacopsychiatry. 23 (Suppl 3): 124–128. doi:10.1055/s-2007-1014548. PMID 1974072.
- Patat A, Perault MC, Vandel B, Danjou P, Brohier S, Zieleniuk I, Rosenzweig P (January 1995). "Assessment of the interaction between a partial agonist and a full agonist of benzodiazepine receptors, based on psychomotor performance and memory, in healthy volunteers". Journal of Psychopharmacology. 9 (2): 91–101. doi:10.1177/026988119500900203. PMID 22298734. S2CID 25269937.
- Baty V, Denis B, Goudot C, Bas V, Renkes P, Bigard MA, et al. (1994). "[Hepatitis induced by alpidem (Ananxyl). Four cases, one of them fatal]" [Hepatitis induced by alpidem (Ananxyl). Four cases, one of them fatal]. Gastroenterologie Clinique et Biologique (in French). 18 (12): 1129–1131. PMID 7750686.
- Ausset P, Malavialle P, Vallet A, Miremont G, Le Bail B, Dumas F, et al. (February 1995). "[Subfulminant hepatitis caused by alpidem and treated by liver transplantation]" [Subfulminant hepatitis caused by alpidem and treated by liver transplantation]. Gastroenterologie Clinique et Biologique (in French). 19 (2): 222–223. PMID 7750714.
- Labbe G, Pessayre D, Fromenty B (August 2008). "Drug-induced liver injury through mitochondrial dysfunction: mechanisms and detection during preclinical safety studies". Fundamental & Clinical Pharmacology. 22 (4): 335–353. doi:10.1111/j.1472-8206.2008.00608.x. PMID 18705745. S2CID 22969724.
- Pessayre D, Mansouri A, Berson A, Fromenty B (2010). "Mitochondrial involvement in drug-induced liver injury". Adverse Drug Reactions. Handbook of Experimental Pharmacology. Vol. 196. pp. 311–365. doi:10.1007/978-3-642-00663-0_11. ISBN 978-3-642-00662-3. PMID 20020267.
- Berson A, Descatoire V, Sutton A, Fau D, Maulny B, Vadrot N, et al. (November 2001). "Toxicity of alpidem, a peripheral benzodiazepine receptor ligand, but not zolpidem, in rat hepatocytes: role of mitochondrial permeability transition and metabolic activation". The Journal of Pharmacology and Experimental Therapeutics. 299 (2): 793–800. PMID 11602696.
- Musch B, Morselli PL, Priore P (April 1988). "Clinical studies with the new anxiolytic alpidem in anxious patients: an overview of the European experiences". Pharmacology, Biochemistry, and Behavior. 29 (4): 803–806. doi:10.1016/0091-3057(88)90211-0. PMID 2901120. S2CID 42445239.
- Cerne R, Lippa A, Poe MM, Smith JL, Jin X, Ping X, et al. (June 2022). "GABAkines - Advances in the discovery, development, and commercialization of positive allosteric modulators of GABAA receptors". Pharmacology & Therapeutics. 234: 108035. doi:10.1016/j.pharmthera.2021.108035. PMC 9787737. PMID 34793859.
- Taliani S, Da Settimo F, Da Pozzo E, Chelli B, Martini C (2009). "Translocator protein ligands as promising therapeutic tools for anxiety disorders". Current Medicinal Chemistry. 16 (26): 3359–3380. doi:10.2174/092986709789057653. PMID 19548867.
- Bourguignon JJ (1993). "Endogenous and Synthetic Ligand of Mitochondrial Benzodiazepine Receptors: Structure-Affinity Relationships.". In Giesen-Crouse E (ed.). Peripheral Benzodiazepine Receptors. London: Academic Press. pp. 59–85.
- Costa E, Romeo E, Auta J, Papadopoulos V, Kozikowski A, Guidotti A (1991). "Is There a Pharmacology of Brain Steroidogenesis? In Neurosteroid and Brain Function". In Costa E, Paul SM (eds.). Fidia ResearchFundation Symposium Series. New York: Thieme Medical Publisher, Inc. pp. 171–176.
- Romeo E, Auta J, Kozikowski AP, Ma D, Papadopoulos V, Puia G, et al. (September 1992). "2-Aryl-3-indoleacetamides (FGIN-1): a new class of potent and specific ligands for the mitochondrial DBI receptor (MDR)". The Journal of Pharmacology and Experimental Therapeutics. 262 (3): 971–978. PMID 1326631.
- Kozikowski AP, Ma D, Romeo E, Auta J, Papadopoulos V, Puia G, Costa E, Guidotti A (August 1992). "Synthesis of (2‐Arylindol‐3‐yl) acetamides as Probes of Mitochondrial Steroidogenesis—A New Mechanism for GABAA Receptor Modulation". Angewandte Chemie International Edition in English. 31 (8): 1060–2. doi:10.1002/anie.199210601.
- Zivkovic B, Arbilla S, Perrault G, Sanger DJ, Langer SZ (September 1991). "Alpidem, an omega-1 receptor-selective partial agonist: a new approach to anxiety treatment". European Neuropsychopharmacology. 1 (3): 202–205. doi:10.1016/0924-977X(91)90485-D. ISSN 0924-977X. S2CID 54414875.
- Sieghart W, Savić MM (October 2018). "International Union of Basic and Clinical Pharmacology. CVI: GABAA Receptor Subtype- and Function-selective Ligands: Key Issues in Translation to Humans". Pharmacological Reviews. 70 (4): 836–878. doi:10.1124/pr.117.014449. PMID 30275042. S2CID 52895644.
- Haefely W, Martin JR, Schoch P (November 1990). "Novel anxiolytics that act as partial agonists at benzodiazepine receptors". Trends in Pharmacological Sciences. 11 (11): 452–456. doi:10.1016/0165-6147(90)90126-s. PMID 1980040.
- Nothdurfter C, Rammes G, Baghai TC, Schüle C, Schumacher M, Papadopoulos V, Rupprecht R (January 2012). "Translocator protein (18 kDa) as a target for novel anxiolytics with a favourable side-effect profile". Journal of Neuroendocrinology. 24 (1): 82–92. doi:10.1111/j.1365-2826.2011.02166.x. PMID 21609361. S2CID 21476596.
- Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N, et al. (December 2010). "Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders". Nature Reviews. Drug Discovery. 9 (12): 971–988. doi:10.1038/nrd3295. PMID 21119734. S2CID 23296183.
- Sanger DJ, Benavides J, Perrault G, Morel E, Cohen C, Joly D, Zivkovic B (1994). "Recent developments in the behavioral pharmacology of benzodiazepine (omega) receptors: evidence for the functional significance of receptor subtypes". Neuroscience and Biobehavioral Reviews. 18 (3): 355–372. doi:10.1016/0149-7634(94)90049-3. PMID 7984354. S2CID 11612995.
- Langer SZ, Faure-Halley C, Seeburg P, Graham D, Arbilla S (September 1992). "The selectivity of zolpidem and alpidem for the α1-subunit of the GABAA receptor". European Neuropsychopharmacology. 2 (3): 232–234. doi:10.1016/0924-977X(92)90081-I. ISSN 0924-977X. S2CID 140210499.
- Richter G, Liao VW, Ahring PK, Chebib M (2020). "The Z-Drugs Zolpidem, Zaleplon, and Eszopiclone Have Varying Actions on Human GABA A Receptors Containing γ1, γ2, and γ3 Subunits". Frontiers in Neuroscience. 14: 599812. doi:10.3389/fnins.2020.599812. PMC 7710685. PMID 33328871.
- Langer SZ, Arbilla S, Tan S, Lloyd KG, George P, Allen J, Wick AE (May 1990). "Selectivity for omega-receptor subtypes as a strategy for the development of anxiolytic drugs". Pharmacopsychiatry. 23 (Suppl 3): 103–107. doi:10.1055/s-2007-1014544. PMID 1974068.
- Pleuvry BJ (August 2004). "Anxiolytics and hypnotics". Anaesthesia & Intensive Care Medicine. 5 (8): 252–256. doi:10.1383/anes.5.8.252.43294. ISSN 1472-0299.
- Guilarte TR (February 2019). "TSPO in diverse CNS pathologies and psychiatric disease: A critical review and a way forward". Pharmacology & Therapeutics. 194: 44–58. doi:10.1016/j.pharmthera.2018.09.003. PMC 6348013. PMID 30189290.
- Witkin JM, Lippa A, Smith JL, Jin X, Ping X, Biggerstaff A, et al. (February 2022). "The imidazodiazepine, KRM-II-81: An example of a newly emerging generation of GABAkines for neurological and psychiatric disorders". Pharmacology, Biochemistry, and Behavior. 213: 173321. doi:10.1016/j.pbb.2021.173321. PMID 35041859. S2CID 245963990.
- Langer SZ, Arbilla S, Benavides J, Scatton B (1990). "Zolpidem and alpidem: two imidazopyridines with selectivity for omega 1- and omega 3-receptor subtypes". Advances in Biochemical Psychopharmacology. 46: 61–72. PMID 1981304.
- Sanger DJ, Perrault G, Morel E, Joly D, Zivkovic B (1991). "Animal models of anxiety and the development of novel anxiolytic drugs". Progress in Neuro-Psychopharmacology & Biological Psychiatry. 15 (2): 205–212. doi:10.1016/0278-5846(91)90082-c. PMID 1678541. S2CID 36709046.
- Bergman J, France CP, Holtzman SG, Katz JL, Koek W, Stephens DN (December 2000). "Agonist efficacy, drug dependence, and medications development: preclinical evaluation of opioid, dopaminergic, and GABAA-ergic ligands". Psychopharmacology. 153 (1): 67–84. doi:10.1007/s002130000567. PMID 11255930. S2CID 1171230.
- Quagliato LA, Carta MG, Nardi AE (2022). "Panic Disorder Seeks More Specific Drugs for Treatment: Might the Amygdala Be the Best Target?". Journal of Clinical Psychopharmacology. 42 (5): 427–428. doi:10.1097/JCP.0000000000001591. PMID 36099401. S2CID 252219658.
- Diamond BI, Nguyen H, O'Neal E, Ochs R, Kaffeman M, Borison RL (1991). "A comparative study of alpidem, a nonbenzodiazepine, and lorazepam in patients with nonpsychotic anxiety". Psychopharmacology Bulletin. 27 (1): 67–71. PMID 1677774.
- Swiss Pharmaceutical Society (2000). Swiss Pharmaceutical Society (ed.). Index Nominum 2000: International Drug Directory. Taylor & Francis. pp. 31–. ISBN 978-3-88763-075-1.
- "US TRIALS OF ALPIDEM HALTED - Pharmaceutical industry news".
Further reading
- Musch B, Morselli PL, Priore P (April 1988). "Clinical studies with the new anxiolytic alpidem in anxious patients: an overview of the European experiences". Pharmacology, Biochemistry, and Behavior. 29 (4): 803–806. doi:10.1016/0091-3057(88)90211-0. PMID 2901120. S2CID 42445239.
- Langer SZ, Arbilla S, Benavides J, Scatton B (1990). "Zolpidem and alpidem: two imidazopyridines with selectivity for omega 1- and omega 3-receptor subtypes". Advances in Biochemical Psychopharmacology. 46: 61–72. PMID 1981304.
- Langer SZ, Arbilla S, Tan S, Lloyd KG, George P, Allen J, Wick AE (May 1990). "Selectivity for omega-receptor subtypes as a strategy for the development of anxiolytic drugs". Pharmacopsychiatry. 23 (Suppl 3): 103–107. doi:10.1055/s-2007-1014544. PMID 1974068.
- Zivkovic B, Morel E, Joly D, Perrault G, Sanger DJ, Lloyd KG (May 1990). "Pharmacological and behavioral profile of alpidem as an anxiolytic". Pharmacopsychiatry. 23 (Suppl 3): 108–113. doi:10.1055/s-2007-1014545. PMID 1974069.
- Morton S, Lader M (May 1990). "Studies with alpidem in normal volunteers and anxious patients". Pharmacopsychiatry. 23 (Suppl 3): 120–123. doi:10.1055/s-2007-1014547. PMID 1974071.
- Hindmarch I (May 1990). "Alpidem and psychological performance in elderly subjects". Pharmacopsychiatry. 23 (Suppl 3): 124–128. doi:10.1055/s-2007-1014548. PMID 1974072.
- Morselli PL (May 1990). "On the therapeutic action of alpidem in anxiety disorders: an overview of the European data". Pharmacopsychiatry. 23 (Suppl 3): 129–134. doi:10.1055/s-2007-1014549. PMID 1974073.
- Zivkovic B, Arbilla S, Perrault G, Sanger DJ, Langer SZ (September 1991). "Alpidem, an omega-1 receptor-selective partial agonist: a new approach to anxiety treatment". European Neuropsychopharmacology. 1 (3): 202–205. doi:10.1016/0924-977X(91)90485-D. ISSN 0924-977X. S2CID 54414875.
- Durand A, Thénot JP, Bianchetti G, Morselli PL (1992). "Comparative pharmacokinetic profile of two imidazopyridine drugs: zolpidem and alpidem". Drug Metabolism Reviews. 24 (2): 239–266. doi:10.3109/03602539208996294. PMID 1576937.
- Sanger DJ, Benavides J, Perrault G, Morel E, Cohen C, Joly D, Zivkovic B (1994). "Recent developments in the behavioral pharmacology of benzodiazepine (omega) receptors: evidence for the functional significance of receptor subtypes". Neuroscience and Biobehavioral Reviews. 18 (3): 355–372. doi:10.1016/0149-7634(94)90049-3. PMID 7984354. S2CID 11612995.
- Skolnick P (November 2012). "Anxioselective anxiolytics: on a quest for the Holy Grail". Trends in Pharmacological Sciences. 33 (11): 611–620. doi:10.1016/j.tips.2012.08.003. PMC 3482271. PMID 22981367.