Neuston
Neuston, also called pleuston, are organisms that live at the surface of a body of water, such as an ocean, estuary, lake, river, or pond. Neuston can live on top of the water surface or may be attached to the underside of the water surface. They may also exist in the surface microlayer that forms between the top side and the underside. Neuston have been defined as "organisms living at the air/water interface of freshwater, estuarine, and marine habitats or referring to the biota on or directly below the water’s surface layer."[1]

The word neuston comes from the Greek neustos, meaning "swimming" + -on (as in plankton).[2] This term first appears in the biological literature in 1917.[3] The alternative term pleuston comes from the Greek plein, meaning "to sail or float". The first known use of this word was in 1909, before the first known use of neuston.[4] In the past various authors have attempted distinctions between neuston and pleuston, but these distinctions have not been widely adopted. As of 2021, the two terms are usually used somewhat interchangeably, and neuston is used more often than pleuston.
Overview
.jpg.webp)
emblematic figure of the marine pleuston
The neuston of the surface layer is one of the lesser known aquatic ecological groups.[5] The term was first used in 1917 by Naumann to describe species associated with the surface layer of freshwater habitats.[3] Later in 1971, Zaitsev identified neuston composition in marine waters.[6] These populations would include microscopic species, plus various plant and animal taxa, such as phytoplankton and zooplankton, living in this region.[6][7] In 2002, Gladyshev further characterised the major physical and chemical dynamics of the surface layer influencing the composition and relationships with various neustonic populations"[8][7]
The neustonic community structure is conditioned by sunlight and an array of endogenous (organic matter, respiratory, photosynthetic, decompositional processes) and exogenous (atmospheric deposition, inorganic matter, winds, wave action, precipitation, UV radiation, oceanic currents, surface temperature) variables and processes affecting nutrient inputs and recycling.[7][9][10] Furthermore, the neuston provides a food source to the zooplankton migrating from deeper layers to the surface,[11] as well as to seabirds roaming over the oceans.[12] For these reasons, the neustonic community is believed to play a critical role on the structure and function of marine food webs. Yet, research on neuston communities to date focused predominantly on geographically-limited regions of the ocean [13][11][14][15][10] or coastal areas.[16][17][18] Consequently, neuston complexity is still poorly understood as studies on the community structure and the taxonomical composition of organisms inhabiting this ecological niche remain few,[10] and global scale analyses are yet lacking.[5]
Types
.jpg.webp)
There are different ways neuston can be categorised. Kennish divides them by their physical position into two groups:[1]
- epineuston: organisms living on the water’s surface
- hyponeuston: organisms within a region of specified depth directly below the surface layer
To this can be added the organisms living in the microlayer at the interface between air and water:
- microlayer neuston: organisms (microorganisms) living in the surface microlayer sandwiched between the upper and under surface.
Marshall and Burchardt divide neuston into three ecological categories:[7][5]
- euneuston: organisms with maximum abundance in the vicinity of the surface on which they reside day and night
- facultative neuston: organisms concentrating at the surface only during certain hours of the day, usually during darkness
- pseudoneuston: organisms with maximum concentrations at deeper layers but reaching the surface layer at least during certain hours.
Freshwater neuston
Freshwater neuston, organisms living at lake or pond surfaces or slow moving parts of rivers and streams, include beetles (see whirligig beetle), protozoans, bacteria and spiders (see fishing spider and diving bell spider). Springtails in the genera Podura and Sminthurides are almost exclusively neustonic, while Hypogastrura species often aggregate on pond surfaces. Water striders such as Gerris are common examples of insects that support their weight on water's surface tension.
Floods

There are different terrestrial environmental factors such as flood pulses and droughts, and these environmental factors affect species such as neuston, whether the effects lead to more or less variations in the species. When flood pulses (an abiotic factor) occur, connectivity between different aquatic environments occur. Species that live in environments with irregular flood patterns tend to have more variations, or even decrease species and variations; similar idea to what happens when droughts occur.[19]
Red fire ants have adapted to contend with both flooding and drought conditions. If the ants sense increased water levels in their nests, they link together and form a ball or raft that floats, with the workers on the outside and the queen inside.[20][21][22] The brood is transported to the highest surface.[23] They are also used as the founding structure of the raft, except for the eggs and smaller larvae. Before submerging, the ants will tip themselves into the water and sever connections with the dry land. In some cases, workers may deliberately remove all males from the raft, resulting in the males drowning.
The longevity of a raft can be as long as 12 days. Ants that are trapped underwater escape by lifting themselves to the surface using bubbles which are collected from submerged substrate.[23] Owing to their greater vulnerability to predators, red imported fire ants are significantly more aggressive when rafting. Workers tend to deliver higher doses of venom, which reduces the threat of other animals attacking. Due to this, and because a higher workforce of ants is available, rafts are potentially dangerous to those that encounter them.[24]
Marine neuston
The marine neuston, organisms living at the ocean surface, are one of the least studied planktonic groups. Neuston occupies a restricted ecological niche and is affected by a wide range of endogenous and exogenous processes while also being a food source to zooplankton and fish migrating from the deep layers and seabirds.[5]
The neustonic animals form a subset of the zooplankton community, which plays a pivotal role in the functioning of marine ecosystems. Zooplankton are partially responsible for the active energy flux between superficial and deep layers of the ocean.[25][26][27] Zooplankton species composition, biomass, and secondary production influence a wide range of trophic levels in marine communities, as they constitute a link between primary production and secondary consumers.[28][29][30] Copepods constitute the most abundant zooplankton taxon in terms of biomass and diversity worldwide.[31][32] Consequently changes in their community composition can impact the biogeochemical cycles [33] and might be indicative of climate variability impacts on ecosystem functioning.[34][5]
 Portuguese man-o-war Physalia sp.
Portuguese man-o-war Physalia sp.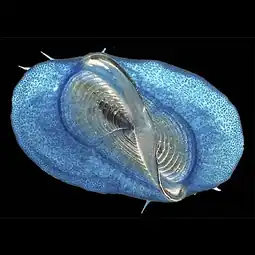 By-the-wind sailor Velella sp.
By-the-wind sailor Velella sp. Blue button Porpita sp.
Blue button Porpita sp. Flying fish from the family Exocoetidae
Flying fish from the family Exocoetidae Buoy barnacle Dosima fascicularis
Buoy barnacle Dosima fascicularis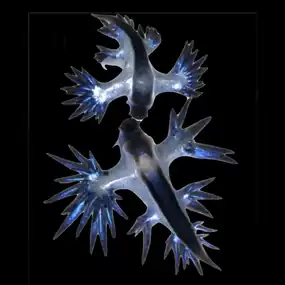 Blue sea dragons Glaucus sp.
Blue sea dragons Glaucus sp.
Historically, zooplankton assemblages research has focused mainly on taxonomic studies and those related to community structure.[35] However, recently, research has veered toward an alternative trait-based approach,[35][29][36] providing a perspective more focused on groups of species with analogous functional traits. This allows individuals to be classified into types characterized by the presence/absence of certain alleles of a gene, into size classes, ecological guilds, or functional groups (FGs).[37] Functional traits are phenotypes affecting organism fitness, growth, survival, and reproductive ability.[38][30] These are regulated by the expression of genes within species, and the expression of traits regulate, in turn, the species fitness under contrasting biotic and abiotic circumstances.[39] Moreover, a specific functional trait can also develop from the interactions between other traits and environmental conditions,[31] leading to a given trait grouping being favoured under certain conditions. Zooplankton traits can be classified in accordance to ecological functions – feeding, growth, reproduction, survival, and other characteristics such as morphology, physiology, behaviour, or life history.[28][40][41] Particularly, feeding strategies and trophic groups are relevant to establish feeding efficiency and associated predation risk.[42] Additionally, they facilitate the understanding of ecosystem services associated with zooplankton, such as the distribution of fisheries or biogeochemical cycling [43] while also allowing the positioning of zooplankton taxa in the food web.[29][44][5]
 Paper nautilus Aurgonaut sp.
Paper nautilus Aurgonaut sp. Sargassum sp. seaweed
Sargassum sp. seaweed Hippolytidae shrimp
Hippolytidae shrimp Marine snail Recluzia sp.
Marine snail Recluzia sp. Violet snail Janthina sp.
Violet snail Janthina sp. Floating anemone Actinecta sp.
Floating anemone Actinecta sp.
Coral-treaders are a genus of quite rare wingless marine bugs known only from coral reefs in the Indo-Pacific region. During low tide they move over water surfaces around coral atolls and reefs similar to the more familiar water-striders, staying submerged in reef crevices during high tide.
See also
References
- Kennish, Michael J., ed. (2016). "Encyclopedia of Estuaries". Encyclopedia of Earth Sciences Series. Dordrecht: Springer Netherlands. doi:10.1007/978-94-017-8801-4. ISBN 978-94-017-8800-7. ISSN 1388-4360. S2CID 129770661.
- Merriam-Webster Dictionary: neuston. Accessed 18 December 2021.
- Naumann, E. (1917) "Beiträge zur Kenntnis des Teichnannoplanktons. II. Über das Neuston des Süsswassers", Biologisches Zentralblatt, 37: 98–106.
- Merriam-Webster Dictionary: pleuston. Accessed 18 December 2021.
- Albuquerque, Rui; Bode, Antonio; González-Gordillo, Juan Ignacio; Duarte, Carlos M.; Queiroga, Henrique (2021). "Trophic Structure of Neuston Across Tropical and Subtropical Oceanic Provinces Assessed with Stable Isotopes". Frontiers in Marine Science. 7. doi:10.3389/fmars.2020.606088.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Zaitsev, Y. P. (1971) "Marine Neustonology". National Marine Fisheries Service, NOAA and NSF, Washington DC.
- Marshall, Harold G.; Burchardt, Lubomira (2005). "Neuston: Its definition with a historical review regarding its concept and community structure". Archiv für Hydrobiologie. 164 (4): 429–448. doi:10.1127/0003-9136/2005/0164-0429.
- Gladyshev, Michail (2002). Biophysics of the surface microlayer of aquatic ecosystems. London: IWA. ISBN 978-1-900222-17-4. OCLC 49871862.
- Barnes, D. K.A.; Davenport, J.; Rawlinson, K. A. (2005). "Temporal Variation in Diversity and Community Structure of a Semi-Isolated Neuston Community". Biology and Environment: Proceedings of the Royal Irish Academy. 105 (2): 107–122. doi:10.3318/bioe.2005.105.2.107. S2CID 84508946.
- Rezai, Hamid; Kabiri, Keivan; Arbi, Iman; Amini, Nafiseh (2019). "Neustonic zooplankton in the northeastern Persian Gulf". Regional Studies in Marine Science. 26: 100473. Bibcode:2019RSMS...2600473R. doi:10.1016/j.rsma.2018.100473. S2CID 135269465.
- Hempel, G. and Weikert, H. (1972) "The neuston of the subtropical and boreal North-eastern Atlantic Ocean. A review". Marine Biology, 13(1): 70–88.
- Cheng, L., Spear, L. and AINLEY, D.G. (2010) "Importance of marine insects (Heteroptera: Gerridae, Halobates spp.) as prey of eastern tropical Pacific seabirds". Marine Ornithology, 38": 91–95.
- Zaitsev, Y. P. (1971). Marine Neustonology. ed. K. A. Vinogradov (Jerulasem: Israel program for scientific translations).
- Holdway, P.; Maddock, L. (1983). "A comparative survey of neuston: Geographical and temporal distribution patterns". Marine Biology. 76 (3): 263–270. doi:10.1007/BF00393027. S2CID 84587026.
- Ebberts, B. D., and Wing, B. L. (1997). "Diversity and abundance of neustonic zooplankton in the North Pacific subarctic frontal zone". U.S. Department of Commerce, NOAA Technical Memorandum NMFS-AFSC-70.
- Brodeur, Richard D. (1989). "Neustonic feeding by juvenile salmonids in coastal waters of the Northeast Pacific". Canadian Journal of Zoology. 67 (8): 1995–2007. doi:10.1139/z89-284.
- Le Fèvre, J.; Bourget, E. (1991). "Neustonic niche for cirripede larvae as a possible adaptation to long-range dispersal". Marine Ecology Progress Series. 75: 185–194. Bibcode:1991MEPS...75..185L. doi:10.3354/meps075185.
- Padmavati, G. and Goswami, S.C. (1996). "Zooplankton distribution in neuston and water column along west coast of India from Goa to Gujarat". Indian J. Mar. Species, 25: 85–90.
- Conceição, E. de O. da, Higuti, J., Campos, R. de, & Martens, K. (2018). Effects of flood pulses on persistence and variability of pleuston communities in a tropical floodplain lake. Hydrobiologia, 807(1), 175–188.
- Mlot, N.J.; Craig, A.T.; Hu, D.L. (2011). "Fire ants self-assemble into waterproof rafts to survive floods". Proceedings of the National Academy of Sciences. 108 (19): 7669–7673. Bibcode:2011PNAS..108.7669M. doi:10.1073/pnas.1016658108. PMC 3093451. PMID 21518911.
- Morrill, W.L. (1974). "Dispersal of red imported fire ants by water". The Florida Entomologist. 57 (1): 39–42. doi:10.2307/3493830. JSTOR 3493830.
- Hölldobler & Wilson 1990, p. 171.
- Adams, B.J.; Hooper-Bùi, L.M.; Strecker, R.M.; O'Brien, D.M. (2011). "Raft formation by the red imported fire ant, Solenopsis invicta". Journal of Insect Science. 11 (171): 171. doi:10.1673/031.011.17101. PMC 3462402. PMID 22950473.
- Haight, K.L. (2006). "Defensiveness of the fire ant, Solenopsis invicta, is increased during colony rafting". Insectes Sociaux. 53 (1): 32–36. doi:10.1007/s00040-005-0832-y. S2CID 24420242.
- Turner, JT (2002). "Zooplankton fecal pellets, marine snow and sinking phytoplankton blooms". Aquatic Microbial Ecology. 27: 57–102. doi:10.3354/ame027057.
- Jónasdóttir, Sigrún Huld; Visser, André W.; Richardson, Katherine; Heath, Michael R. (2015). "Seasonal copepod lipid pump promotes carbon sequestration in the deep North Atlantic". Proceedings of the National Academy of Sciences. 112 (39): 12122–12126. doi:10.1073/pnas.1512110112. PMC 4593097. PMID 26338976.
- Hernández-León, S.; Koppelmann, R.; Fraile-Nuez, E.; Bode, A.; Mompeán, C.; Irigoien, X.; Olivar, M. P.; Echevarría, F.; Fernández De Puelles, M. L.; González-Gordillo, J. I.; Cózar, A.; Acuña, J. L.; Agustí, S.; Duarte, C. M. (2020). "Large deep-sea zooplankton biomass mirrors primary production in the global ocean". Nature Communications. 11 (1): 6048. Bibcode:2020NatCo..11.6048H. doi:10.1038/s41467-020-19875-7. PMC 7695708. PMID 33247160. S2CID 227191974.
- Litchman, Elena; Ohman, Mark D.; Kiørboe, Thomas (2013). "Trait-based approaches to zooplankton communities". Journal of Plankton Research. 35 (3): 473–484. doi:10.1093/plankt/fbt019.
- Benedetti, Fabio; Gasparini, Stéphane; Ayata, Sakina-Dorothée (2016). "Identifying copepod functional groups from species functional traits". Journal of Plankton Research. 38 (1): 159–166. doi:10.1093/plankt/fbv096. PMC 4722884. PMID 26811565.
- Sodré, Elder de Oliveira; Bozelli, Reinaldo Luiz (2019). "How planktonic microcrustaceans respond to environment and affect ecosystem: A functional trait perspective". International Aquatic Research. 11 (3): 207–223. doi:10.1007/s40071-019-0233-x. S2CID 197594398.
- Kiørboe, Thomas (2011). "How zooplankton feed: Mechanisms, traits and trade-offs". Biological Reviews. 86 (2): 311–339. doi:10.1111/j.1469-185X.2010.00148.x. PMID 20682007. S2CID 25218654.
- Neumann-Leitão, Sigrid; Melo, Pedro A. M. C.; Schwamborn, Ralf; Diaz, Xiomara F. G.; Figueiredo, Lucas G. P.; Silva, Andrea P.; Campelo, Renata P. S.; Melo Júnior, Mauro de; Melo, Nuno F. A. C.; Costa, Alejandro E. S. F.; Araújo, Moacyr; Veleda, Dóris R. A.; Moura, Rodrigo L.; Thompson, Fabiano (2018). "Zooplankton from a Reef System Under the Influence of the Amazon River Plume". Frontiers in Microbiology. 9: 355. doi:10.3389/fmicb.2018.00355. PMC 5838004. PMID 29545783.
- Bianchi, Daniele; Mislan, K. A. S. (2016). "Global patterns of diel vertical migration times and velocities from acoustic data". Limnology and Oceanography. 61 (1): 353–364. Bibcode:2016LimOc..61..353B. doi:10.1002/lno.10219. S2CID 3400176.
- Hooff, Rian C.; Peterson, William T. (2006). "Copepod biodiversity as an indicator of changes in ocean and climate conditions of the northern California current ecosystem". Limnology and Oceanography. 51 (6): 2607–2620. Bibcode:2006LimOc..51.2607H. doi:10.4319/lo.2006.51.6.2607. S2CID 16280978.
- Pomerleau, Corinne; Sastri, Akash R.; Beisner, Beatrix E. (2015). "Evaluation of functional trait diversity for marine zooplankton communities in the Northeast subarctic Pacific Ocean". Journal of Plankton Research. 37 (4): 712–726. doi:10.1093/plankt/fbv045.
- Campos, C.C.; Garcia, T.M.; Neumann-Leitão, S.; Soares, M.O. (2017). "Ecological indicators and functional groups of copepod assemblages". Ecological Indicators. 83: 416–426. doi:10.1016/j.ecolind.2017.08.018.
- Tuomisto, Hanna (2010). "A diversity of beta diversities: Straightening up a concept gone awry. Part 1. Defining beta diversity as a function of alpha and gamma diversity". Ecography. 33: 2–22. doi:10.1111/j.1600-0587.2009.05880.x.
- Violle, Cyrille; Navas, Marie-Laure; Vile, Denis; Kazakou, Elena; Fortunel, Claire; Hummel, Irène; Garnier, Eric (2007). "Let the concept of trait be functional!". Oikos. 116 (5): 882–892. doi:10.1111/j.2007.0030-1299.15559.x.
- Barton, Andrew D.; Pershing, Andrew J.; Litchman, Elena; Record, Nicholas R.; Edwards, Kyle F.; Finkel, Zoe V.; Kiørboe, Thomas; Ward, Ben A. (2013). "The biogeography of marine plankton traits". Ecology Letters. 16 (4): 522–534. doi:10.1111/ele.12063. PMID 23360597.
- Hunt, B.P.V.; Allain, V.; Menkes, C.; Lorrain, A.; Graham, B.; Rodier, M.; Pagano, M.; Carlotti, F. (2015). "A coupled stable isotope-size spectrum approach to understanding pelagic food-web dynamics: A case study from the southwest sub-tropical Pacific". Deep Sea Research Part II: Topical Studies in Oceanography. 113: 208–224. Bibcode:2015DSRII.113..208H. doi:10.1016/j.dsr2.2014.10.023.
- Brun, Philipp; Payne, Mark R.; Kiørboe, Thomas (2016). "Trait biogeography of marine copepods - an analysis across scales" (PDF). Ecology Letters. 19 (12): 1403–1413. doi:10.1111/ele.12688. PMID 27726281. S2CID 216070768.
- Brun, Philipp; Payne, Mark R.; Kiørboe, Thomas (2017). "A trait database for marine copepods". Earth System Science Data. 9 (1): 99–113. Bibcode:2017ESSD....9...99B. doi:10.5194/essd-9-99-2017. S2CID 55732646.
- Prowe, A. E. Friederike; Visser, André W.; Andersen, Ken H.; Chiba, Sanae; Kiørboe, Thomas (2019). "Biogeography of zooplankton feeding strategy". Limnology and Oceanography. 64 (2): 661–678. Bibcode:2019LimOc..64..661P. doi:10.1002/lno.11067. S2CID 91541174.
- Benedetti, Fabio; Vogt, Meike; Righetti, Damiano; Guilhaumon, François; Ayata, Sakina-Dorothée (2018). "Do functional groups of planktonic copepods differ in their ecological niches?" (PDF). Journal of Biogeography. 45 (3): 604–616. doi:10.1111/jbi.13166. S2CID 90358144.
External links
- "neuston - Britannica Online". Encyclopædia Britannica. Retrieved 2007-11-13.