OmpT
OmpT is an aspartyl protease found on the outer membrane of Escherichia coli. OmpT is a subtype of the family of omptin proteases, which are found on some gram-negative species of bacteria.[2]
| Protease 7 | |||||||
|---|---|---|---|---|---|---|---|
 | |||||||
| Identifiers | |||||||
| Organism | |||||||
| Symbol | OmpT | ||||||
| UniProt | P09169 | ||||||
| |||||||
| Outer membrane protease, Plasmid F | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Organism | |||||||
| Symbol | OmpP | ||||||
| UniProt | P34210 | ||||||
| |||||||
Structure
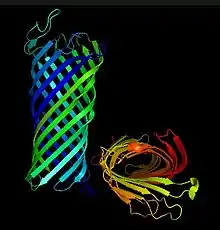
OmpT is a 33.5 kDa outer membrane protein consisting of 10 antiparallel strands that are connected by 5 extracellular loops. The antiparallel strands form a beta barrel structure that spans the width of the membrane, creating a pore.[1]
E. coli omptins can be coded either from the OmpT gene on a chromosome (part of a DLP12 prophage) or from OmpP on a plasmid (OmpP). The sequences resulting from these two sources differ by 24-25% in the mature protease.[3]
Genetic differences between OmpT and other members of the omptin family are found in the extracellular loops, and therefore, this area is thought to be associated with substrate specificity.[2] Also, the barrel is relatively rigid, while the loops have more flexibility to bind to substrates of varying sizes.[4]
Mechanism
While originally thought to be a serine protease, OmpT is better characterized as an aspartyl protease because of its cleavage mechanism.[1]
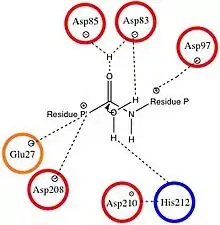
The substrate of OmpT binds to negatively charged aspartate and glutamate residues, so the active site of the protease is anionic. This causes OmpT to selectively cleave peptides between two basic (positively charged) residues. The active site of OmpT resembles that of other omptins, and is characterized by conserved residues at Asp84, Asp86, Asp206, and His208.[5] The most common bond cleavage by OmpT is between two arginine residues because their positive charge can favorably interact with the negatively charged species at the active site during substrate binding.[6]
Because of the specificity of the active site, OmpT does not act on peptides with a negatively charged residue adjacent to the scissile bond.[7] Also, OmpT is specifically identified an endopeptidase because it does not cleave peptides at the N- or C-terminus, but only between nonterminal amino acids.[8]
The peptide bond cleavage occurs via the nucleophilic attack of water at the carbonyl between two adjacent amino acid residues. Water enters the protease from the intracellular surface and is stabilized by Asp83 and His212.[4] During the proton transfer associated with the peptide cleavage, the negatively charged aspartate residue stabilizes the positively charged histidine.[1] Once docked in this position, water is positioned to attack the peptide in the active site.
The cleavage of peptide bonds by OmpT is also dependent on the presence of bound lipopolysaccharide (LPS). When LPS is not present, the peptide binds too deeply within the active site, and the water cannot reach the carbonyl for its nucleophilic attack of the scissile bond.[5]
Biological function and disease relevance
In E. coli, OmpT is a housekeeping protease that degrades foreign peptide material that the bacteria encounters.[9] Because of its ability to cleave peptides present in its surrounding environment, OmpT is associated with several pathologies.
Urinary tract infections
Urinary tract infections (UTIs) are often due to E. coli entering the urethra and colonizing. The host's immune system will release protamines and other antimicrobials to combat the infection, but OmpT easily degrades the cationic protamine peptides, thus enhancing the risk of infection.[10] There is a genetic link between OmpT and other UTI-mediating factors (such as kpsMT, cnf1, prf, and sfa), but the functional link between these proteins is not well defined.[11]
Intestinal colonization and sepsis
Enterohemorrhagic E. coli (EHEC) and enteropathogenic E. coli (EPEC) are pathogens that rely on OmpT to colonize in the intestine of their host. In response to the presence of E. coli in the gut, the host releases antimicrobial peptides as part of the innate immune response. Since OmpT can break down these antimicrobials and inactivate them, EHEC and EPEC can colonize within the colon or small intestine of the host and lead to serious diarrheal diseases.[12]
In the case of sepsis, the host activates the blood clotting system to deposit fibrin and limit the spread of bacteria throughout the blood. However, OmpT can inactivate the tissue factor pathway inhibitor (TFPI), counteracting the host's immune response, and further perpetuating the spread of extraintestinal E. coli infection.[13]
Evolved suicidal action of OmpT
In zebrafish, ZF-RNase-3 (A5HAK0) must be cleaved by a protease (such as OmpT) in order to become activated and serve its bactericidal function.[14] Through this evolved suicidal mechanism, the RNase mediates its own activation, since it is only cleaved in the presence of its bacterial target.
Other applications
OmpT has been identified as a potential probe to use in mass spectrometry-based proteomics, because its substrate specificity allows it to differentiate between proteins with related primary sequences.[15]
References
- Vandeputte-Rutten L, Kramer RA, Kroon J, Dekker N, Egmond MR, Gros P (September 2001). "Crystal structure of the outer membrane protease OmpT from Escherichia coli suggests a novel catalytic site". EMBO J. 20 (18): 5033–9. doi:10.1093/emboj/20.18.5033. PMC 125623. PMID 11566868.
- Yun TH, Morrissey JH (October 2009). "Polyphosphate and omptins: novel bacterial procoagulant agents". J. Cell. Mol. Med. 13 (10): 4146–53. doi:10.1111/j.1582-4934.2009.00884.x. PMC 2891932. PMID 19725923.
- Haiko J, Laakkonen L, Juuti K, Kalkkinen N, Korhonen TK (September 2010). "The omptins of Yersinia pestis and Salmonella enterica cleave the reactive center loop of plasminogen activator inhibitor 1". J. Bacteriol. 192 (18): 4553–61. doi:10.1128/JB.00458-10. PMC 2937412. PMID 20639337.
- Baaden M, Sansom MS (November 2004). "OmpT: molecular dynamics simulations of an outer membrane enzyme". Biophys. J. 87 (5): 2942–53. Bibcode:2004BpJ....87.2942B. doi:10.1529/biophysj.104.046987. PMC 1304768. PMID 15315948.
- Eren E, van den Berg B (July 2012). "Structural basis for activation of an integral membrane protease by lipopolysaccharide". J. Biol. Chem. 287 (28): 23971–6. doi:10.1074/jbc.M112.376418. PMC 3390672. PMID 22645135.
- Hwang BY, Varadarajan N, Li H, Rodriguez S, Iverson BL, Georgiou G (January 2007). "Substrate specificity of the Escherichia coli outer membrane protease OmpP". J. Bacteriol. 189 (2): 522–30. doi:10.1128/JB.01493-06. PMC 1797397. PMID 17085556.
- Dekker N, Cox RC, Kramer RA, Egmond MR (February 2001). "Substrate specificity of the integral membrane protease OmpT determined by spatially addressed peptide libraries". Biochemistry. 40 (6): 1694–701. doi:10.1021/bi0014195. PMID 11327829.
- Sugimura K, Nishihara T (December 1988). "Purification, characterization, and primary structure of Escherichia coli protease VII with specificity for paired basic residues: identity of protease VII and OmpT". J. Bacteriol. 170 (12): 5625–32. doi:10.1128/jb.170.12.5625-5632.1988. PMC 211661. PMID 3056908.
- Haiko J, Suomalainen M, Ojala T, Lähteenmäki K, Korhonen TK (April 2009). "Invited review: Breaking barriers--attack on innate immune defences by omptin surface proteases of enterobacterial pathogens". Innate Immun. 15 (2): 67–80. doi:10.1177/1753425909102559. PMID 19318417.
- Stumpe S, Schmid R, Stephens DL, Georgiou G, Bakker EP (August 1998). "Identification of OmpT as the protease that hydrolyzes the antimicrobial peptide protamine before it enters growing cells of Escherichia coli". J. Bacteriol. 180 (15): 4002–6. doi:10.1128/JB.180.15.4002-4006.1998. PMC 107389. PMID 9683502.
- Foxman B, Zhang L, Palin K, Tallman P, Marrs CF (June 1995). "Bacterial virulence characteristics of Escherichia coli isolates from first-time urinary tract infection". J. Infect. Dis. 171 (6): 1514–21. doi:10.1093/infdis/171.6.1514. PMID 7769286.
- Thomassin JL, Brannon JR, Gibbs BF, Gruenheid S, Le Moual H (February 2012). "OmpT outer membrane proteases of enterohemorrhagic and enteropathogenic Escherichia coli contribute differently to the degradation of human LL-37". Infect. Immun. 80 (2): 483–92. doi:10.1128/IAI.05674-11. PMC 3264287. PMID 22144482.
- Yun TH, Cott JE, Tapping RI, Slauch JM, Morrissey JH (January 2009). "Proteolytic inactivation of tissue factor pathway inhibitor by bacterial omptins". Blood. 113 (5): 1139–48. doi:10.1182/blood-2008-05-157180. PMC 2635079. PMID 18988866.
- Zanfardino A, Pizzo E, Di Maro A, Varcamonti M, D'Alessio G (April 2010). "The bactericidal action on Escherichia coli of ZF-RNase-3 is triggered by the suicidal action of the bacterium OmpT protease". FEBS J. 277 (8): 1921–8. doi:10.1111/j.1742-4658.2010.07614.x. PMID 20214681. S2CID 212827.
- Wu C, Tran JC, Zamdborg L, et al. (August 2012). "A protease for 'middle-down' proteomics". Nat. Methods. 9 (8): 822–4. doi:10.1038/nmeth.2074. PMC 3430368. PMID 22706673.