Orellanine
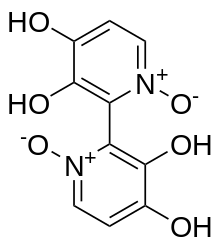
Orellanine or orellanin is a mycotoxin found in a group of mushrooms known as the Orellani of the family Cortinariaceae.[1] Structurally, it is a bipyridine N-oxide compound somewhat related to the herbicide diquat.
 | |
| Names | |
|---|---|
| IUPAC name
3,3′,4,4′-Tetrahydroxy-2,2′-bipyridine-N,N′-dioxide | |
| Other names
Orellanin, 2,2-Bipyridine-3,3-4,4-tetrol-1,1-dioxide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.232.424 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H8N2O6 | |
| Molar mass | 252.182 g·mol−1 |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Highly toxic with delayed onset of toxicity |
| GHS labelling: | |
  | |
| Danger | |
| H300, H370 | |
| P260, P264, P270, P301+P310, P307+P311, P321, P330, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
History
Orellanine first came to people's attention in 1952 when a mass poisoning of 102 people in Konin, Poland, resulted in 11 deaths.[2] Orellanine comes from a class of mushrooms that fall under the genus Cortinarius, and has been found in the species C. orellanus, rubellus, henrici, rainerensis and bruneofulvus.[2][3] Poisonings related to these mushrooms have occurred predominately in Europe where mushroom foraging was common, though cases of orellanine poisoning have been reported in North America and Australia as well.[3] There are several reported cases of people ingesting orellanine-containing mushrooms after mistaking them for edible or hallucinogenic mushrooms.[3][4]
Orellanine was first isolated in 1962, when Stanisław Grzymala extracted and isolated orellanine from the mushroom C. orellanus.[3][5] Grzymala was also able to demonstrate the nephrotoxicity of C. orellanus and determine various physical and chemical properties of orellanine. He found that the toxicity of the mushroom was due to both delayed and acute kidney injury.
The chemical structure of orellanine was first deduced by Antkowiak and Gessner in 1979, who identified it as 3,3',4,4'-tetrahydroxy- 2,2'-bipyridine-1,1'-dioxide.[3][6]
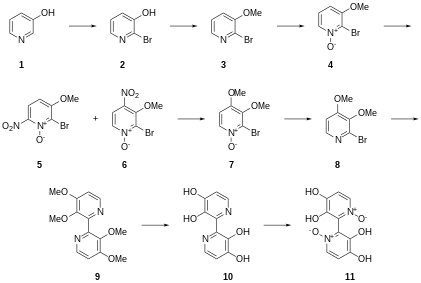
The first successful synthesis of orellanine was reported in 1985.[7] The total synthesis of orellanine from 3-hydroxypyridine was reported a year later in 1986.[8]
Synthesis
The first synthesis of orellanine was reported in 1985 by Dehmlow and Schulz, and required ten steps starting from 3-aminopyridine.[7] The following year, Tiecco et al. reported the total synthesis of orellanine in nine steps starting from 3-hydroxypyridine.[8]
Structure
Orellanine is a bipyridine N-oxide.[3][6] Orellanine displays tautomerism, with the more stable tautomer being the pyridine N-oxide form.[3][6]
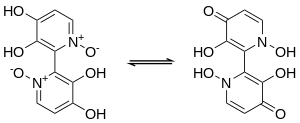
The chemical structure of orellanine has been confirmed by X-ray crystallography.[9][10] In the crystal structure, the two pyridine rings are nearly perpendicular to each other, making orellanine chiral.[9][10] However, samples of orellanine extracted from mushrooms are optically inactive racemic mixtures, likely due to racemization during the extraction process.[3][10]
Toxicity
Orellanine displays a wide spectrum of toxin effects in plants, animals, and microorganisms.[3] Although the mechanism of toxicity of orellanine is not yet fully understood, it likely targets cellular processes found in both prokaryotes and eukaryotes.[3] Orellanine has been found to inhibit the synthesis of biomolecules such as proteins, RNA, and DNA, and promote non-competitive inhibition of several enzymes such as alkaline phosphatase, γ-glutamyltransferase, and leucyl aminopeptidase.[3] In addition, orellanine has also been shown to interfere with the production of adenosine triphosphatase.[3]
Orellanine is a bipyridine with positively charged nitrogen atoms, and chemically resembles the bipyridine herbicides paraquat and diquat.[3] Like orellanine, paraquat and diquat are toxic not only to plants, but also to humans and livestock. Bipyridine compounds with charged nitrogen atoms disrupt important redox reactions in organisms, 'stealing' one or two electrons and sometimes passing the electrons along into other, often undesirable, redox reactions. The terminal products of these reactions can be harmful reactive oxygen species such as peroxide or superoxide ions, the latter of which are harmful to cells. It is thought that orellanine produces oxidative stress in a similar manner to paraquat and diquat.[3]
In humans, a characteristic of poisoning by the nephrotoxin orellanine is the long latency; the first symptoms usually do not appear until 2–4 to 14 days after ingestion.[3] The latent period decreases with the quantity of mushrooms consumed.[3] The first symptoms of orellanine poisoning are similar to the common flu (nausea, vomiting, stomach pains, headaches, myalgia, etc.), these symptoms are followed by early stages of kidney failure (immense thirst, frequent urination, pain on and around the kidneys) and eventually decreased or nonexistent urine output and other symptoms of kidney failure occur. If left untreated death will follow.
The LD50 of orellanine in mice is 12 to 20 mg per kg body weight;[11][12] this is the dose which leads to death within two weeks. From cases of orellanine-related mushroom poisoning in humans it seems that the lethal dose for humans is considerably lower.
Treatment
There is no known antidote against orellanine poisoning. Treatment consists mainly of supportive care and hemodialysis, if needed.[3] Complete recovery of renal function is recovered in only 30% of poisoned patients.[3] There are reports of cases where treatment using corticosteroids and antioxidants led to improved clinical outcomes.[3]
See also
References
- Oubrahim H.; Richard J.-M.; Cantin-Esnault D.; Seigle-Murandi F.; Trecourt F (1997). "Novel methods for identification and quantification of the mushroom nephrotoxin orellanine". Journal of Chromatography. 758 (1): 145–157. doi:10.1016/S0021-9673(96)00695-4. PMID 9181972.
- Schumacher, Trond; Høiland, Klaus (1983-06-01). "Mushroom poisoning caused by species of the genus Cortinarius fries". Archives of Toxicology. 53 (2): 87–106. doi:10.1007/BF00302720. ISSN 1432-0738. PMID 6349583. S2CID 29016554.
- Dinis-Oliveira, Ricardo Jorge; Soares, Mariana; Rocha-Pereira, Carolina; Carvalho, Félix (2015). "Human and experimental toxicology of orellanine". Human & Experimental Toxicology. 35 (9): 1016–1029. doi:10.1177/0960327115613845. PMID 26553321. S2CID 7230959.
- Deutsch, Christopher J.; Swallow, Daniel (24 September 2012). "Deliberate ingestion of "magic" mushrooms may also cause renal failure". BMJ. 345: e6388. doi:10.1136/bmj.e6388. ISSN 1756-1833. PMID 23008206. S2CID 32430024.
- Gryzmala, S. (1962). "L'isolement de l'Orellanine poison du Cortinarius orellanus Fries et l'étude de ses effets anatomo-pathologiques". Bulletin de la Société Mycologique de France. 78: 394–404.
- Antkowiak, Wieław Z.; Gessner, Wiesł P. (1979-01-01). "The structures of orellanine and orelline". Tetrahedron Letters. 20 (21): 1931–1934. doi:10.1016/S0040-4039(01)86882-9. ISSN 0040-4039.
- Dehmlow, Eckehard V.; Schulz, Hans-Joachim (1985). "Synthesis of orellanine the lethal poison of a toadstool - ScienceDirect". Tetrahedron Letters. 26 (40): 4903–4906. doi:10.1016/S0040-4039(00)94981-5.
- Tiecco, Marcello; Tingoli, Macro; Testaferri, Lorenzo; Chianelli, Donatella; Wenkert, Ernest (1986). "Total synthesis of orellanine : The lethal toxin of Cortinarius orellanus fries mushroom". Tetrahedron. 42 (5): 1475–1485. doi:10.1016/S0040-4020(01)87367-1.
- Kubicki, Maciej; Borowiak, Teresa; Antkowiak, Wiesław Z. (1991-08-01). "Crystal structure of orellanine hydrate". Journal of Crystallographic and Spectroscopic Research. 21 (4): 401–405. doi:10.1007/BF01160652. ISSN 1572-8854. S2CID 94292766.
- Cohen-Addad, C.; Richard, J.-M.; Guitel, J.-C. (1987-03-15). "Structure of an orellanine–trifluoroacetic acid complex: evidence of a very short O–H⋯O hydrogen bond". Acta Crystallographica Section C: Crystal Structure Communications. 43 (3): 504–507. doi:10.1107/S0108270187095222. ISSN 0108-2701.
- Prast, Helmut; Werner; Pfaller; Moser (1988). "Toxic properties of the mushroom Cortinarius orellanus. I. Chemical characterization of the main toxin of Cortinarius orellanus (Fries) and Cortinarius speciosissimus (Kuhn & Romagn) and acute toxicity in mice". Archives of Toxicology. 62 (1): 81–88. doi:10.1007/bf00316263. PMID 3190463. S2CID 24495871.
- Richard, Jean-Michel; Louis, Josette; Cantin, Danielle (1988-03-01). "Nephrotoxicity of orellanine, a toxin from the mushroomCortinarius orellanus". Archives of Toxicology. 62 (2): 242–245. doi:10.1007/BF00570151. ISSN 1432-0738. PMID 3196164. S2CID 36515278.
External links
- Cortinarius rubellus Pacific Northwest Fungi, Featured Fungus Number 4