Oxime V
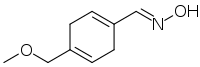
Oxime V is a chemical compound that has been studied as a potential sweetener. Oxime V was first reported in 1976 as a synthetic analog of the artificial sweetener perillartine.[1] It is about 450 times as sweet as sucrose and is more water-soluble than perillartine.[2] Its metabolism and toxicology have been investigated,[3] and it has been found to have promising properties,[2] but it is not currently marketed.
 | |
| Names | |
|---|---|
| IUPAC name
4-(Methoxymethyl)-1,4-cyclohexadiene-1-carboxaldehyde syn-oxime | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
| Properties | |
| C9H13NO2 | |
| Molar mass | 167.208 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- Acton, E. M.; Stone, H. (1976). "Potential New Artificial Sweetener from Study of Structure-Taste Relationships". Science. 193 (4253): 584–586. Bibcode:1976Sci...193..584A. doi:10.1126/science.959816. PMID 959816.
- A. Douglas Kinghorn and Cesar M. Comadre (2001). "Chapter 12. Less Common High-Potency Sweeteners". In Lyn O'Brien-Nabors (ed.). Alternative Sweeteners (3rd ed.). p. 222. ISBN 0-8247-0437-1.
- Hitoma, C.; Acton, E. M.; Degraw, J. I.; Thomas, D. W. (1985). "Metabolic and Toxicologic Study of an Artificial Sweetener, Oxime V". Drug and Chemical Toxicology. 8 (4): 195–206. doi:10.3109/01480548509038645. PMID 3841048.
- Wang, Zhixin; Gmitter, Frederick G.; Grosser, Jude W.; Wang, Yu (2022). "Natural Sweeteners and Sweetness-Enhancing Compounds Identified in Citrus Using an Efficient Metabolomics-Based Screening Strategy". Journal of Agricultural and Food Chemistry. 70 (34): 10593–10603. doi:10.1021/acs.jafc.2c03515. PMID 35980814. S2CID 251645690.
- "Researchers find new sugar substitutes in citrus that could change food and beverage industry". Science Daily. September 20, 2022.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.