Oxohalide
In chemistry, molecular oxohalides (oxyhalides) are a group of chemical compounds in which both oxygen and halogen atoms are attached to another chemical element A in a single molecule. They have the general formula AOmXn, where X is a halogen. Known oxohalides have fluorine (F), chlorine (Cl), bromine (Br), and/or iodine (I) in their molecules. The element A may be a main group element, a transition element, a rare earth element or an actinide. The term oxohalide, or oxyhalide, may also refer to minerals and other crystalline substances with the same overall chemical formula, but having an ionic structure.
Synthesis

Oxohalides can be seen as compounds intermediate between oxides and halides. There are three general methods of synthesis:[1]
- Partial oxidation of a halide:
- 2 PCl3 + O2 → 2 POCl3
- In this example, the oxidation state increases by two and the electrical charge is unchanged.
- Partial halogenation of an oxide:
- 2 V2O5 + 6 Cl2 + 3 C → 4 VOCl3 + 3 CO2
- Oxide replacement:
- CrO2−4 + 2 Cl− + 4 H+ → CrO2Cl2 + 4 H2O
In addition, various oxohalides can be made by halogen exchange reactions and this reaction can also lead to the formation of mixed oxohalides such as POFCl2 and CrO2FCl.
Properties
In relation to the oxide or halide, for a given oxidation state of an element A, if two halogen atoms replace one oxygen atom, or vice versa, the overall charge on the molecule is unchanged and the coordination number of the central atom decreases by one. For example, both phosphorus oxychloride (POCl3) and phosphorus pentachloride, (PCl5) are neutral covalent compounds of phosphorus in the +5 oxidation state. If an oxygen atom is simply replaced by a halogen atom the charge increases by +1, but the coordination number is unchanged. This is illustrated by the reaction of a mixture of a chromate or dichromate salt and potassium chloride with concentrated sulfuric acid.
- Cr2O2−7 + 4 Cl− + 6 H+ → 2 CrO2Cl2 + 3 H2O
The chromyl chloride produced has no electrical charge and is a volatile covalent molecule that can be distilled out of the reaction mixture.[2]
Oxohalides of elements in high oxidation states are strong oxidizing agents, with oxidizing power similar to the corresponding oxide or halide. Most oxohalides are easily hydrolyzed. For example, chromyl chloride is hydrolyzed to chromate in the reverse of the synthetic reaction, above. The driving force for this reaction is the formation of A-O bonds which are stronger than A-Cl bonds. This gives a favourable enthalpy contribution to the Gibbs free energy change for the reaction[3]
Many oxohalides can act as Lewis acids. This is particularly so with oxohalides of coordination number 3 or 4 which, in accepting one or more electron pairs from a Lewis base, become 5- or 6-coordinate. Oxohalide anions such as [VOCl4]2− can be seen as acid-base complexes of the oxohalide (VOCl2) with more halide ions acting as Lewis bases. Another example is VOCl2 which forms the trigonal bipyramidal complex VOCl2(N(CH3)3)2 with the base trimethylamine.[4]
The vibrational spectra of many oxohalides have been assigned in detail. They give useful information on relative bond strengths. For example, in CrO2F2, the Cr–O stretching vibrations are at 1006 cm−1 and 1016 cm−1 and the Cr–F stretching vibrations are at 727 cm−1 and 789 cm−1. The difference is much too large to be due to the different masses of O and F atoms. Rather, it shows that the Cr–O bond is much stronger than the Cr–F bond. M–O bonds are generally considered to be double bonds and this is backed up by measurements of M–O bond lengths. It implies that the elements A and O are chemically bound together by a σ bond and a π bond.[5]
Oxohalides of elements in high oxidation states are intensely coloured owing to ligand to metal charge transfer (LMCT) transitions.[6]
Main group elements
Carbon group
Carbon forms oxohalides COX2, X = F, Br, and the very toxic phosgene (X = Cl), which is produced industrially by a carbon-catalyzed reaction of carbon monoxide with chlorine. It is a useful reagent in organic chemistry for the formation of carbonyl compounds.[7] For example:
- COCl2 + 2 ROH → CO(OR)2 + 2 HCl
Silicon tetrafluoride reacts with water to yield poorly-characterized oxyfluoride polymers, but slow and careful reaction at -196 °C yields the oxyfluoride hexafluorodisiloxane as well.[8]
Pnictogens
Nitrogen forms two series of oxohalides with nitrogen in oxidation states 3, NOX, X = F, Cl, Br and 5, NO2X, X = F, Cl. They are made by halogenation of nitrogen oxides. Note that NO2F is isoelectronic with the nitrate ion, NO−3. Only oxohalides of phosphorus(V) are known.[9]
Chalcogens
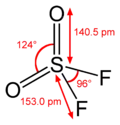
Sulfur forms oxohalides[10] in oxidation state +4, such as thionyl chloride, SOCl2 and oxidation state +6, such as sulfuryl fluoride (SO2F2), sulfuryl chloride (SO2Cl2), and thionyl tetrafluoride (SOF4). All are easily hydrolyzed. Indeed, thionyl chloride can be used as a dehydration agent as the water molecules are converted into gaseous products, leaving behind the anhydrous solid chloride.[11]
- MgCl2·6H2O + 6 SOCl2 → MgCl2 + 6 SO2 + 12 HCl
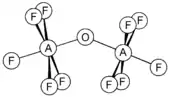
Selenium and tellurium form similar compounds and also the oxo-bridged species F5AOAF5 (A = S, Se, Te). They are non-linear with the A-O-A angle of 142.5, 142.4 and 145.5° for S, Se and Te, respectively.[12] The tellurium anion F5TeO−, known as teflate, is a large and rather stable anion, useful for forming stable salts with large cations.[11]
Halogens
The halogens form various oxofluorides with formulae XO2F (chloryl fluoride), XO3F (perchloryl fluoride) and XOF3 with X = Cl, Br and I. IO2F3 and IOF5 are also known.[13]
Noble gases
Xenon forms xenon oxytetrafluoride (XeOF4), xenon dioxydifluoride (XeO2F2) and xenon oxydifluoride (XeOF2).
Transition metals and actinides
4.png.webp)
A selection of known oxohalides of transition metals is shown below, and more detailed lists are available in the literature.[15] X indicates various halides, most often F and Cl.
| Oxidation state | oxohalides |
|---|---|
| 3 | VOCl, VOBr,[16] FeOCl |
| 4 | [TiOCl4]2−, Cl3TiOTiCl3, VOCl2, [VOCl4]2− |
| 5 | VOX3, VO2F, [CrOF4]−, [CrOF5]2−, MnOCl3, TcOCl3, VOF3, VOCl3, NbOCl3 |
| 6 | CrO2Cl2, [CrO3Cl]−, ReOX4, ReO2F2, OsOF4, CrO2F2, MoOF4, MoOCl4, MoO2Cl2, MoO2F2, WO2Cl2, WO2F2, WOF4, WOCl4 |
| 7 | MnO3F, ReOF5, ReO2F3, ReO3F, ReO3Cl,ReO3Cl, OsOF5 |
| 8 | OsO2F4, OsO3F2 |
High oxidation states of the metal are dictated by the fact that oxygen is a strong oxidizing agent, as is fluorine. Bromine and iodine are relatively weak oxidizing agents, so fewer oxobromides and oxoiodides are known. Structures for compounds with d0 configuration are predicted by VSEPR theory. Thus, CrO2Cl2 is tetrahedral, OsO3F2 is trigonal bipyramidal, XeOF4 is square pyramidal and OsOF5 is octahedral.[18] The d1 complex ReOCl4 is square pyramidal.
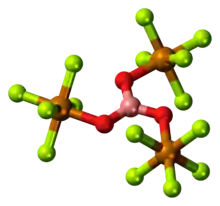
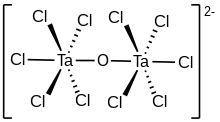
The compounds [Ta2OX10]2− and [M2OCl10]4− (M = W, Ru, Os) have two MX5 groups joined by a bridging oxygen atom.[19] Each metal has an octahedral environment. The unusual linear M−O−M structure can be rationalized in terms of molecular orbital theory, indicating the presence of dπ — pπ bonding between the metal and oxygen atoms.[20] Oxygen bridges are present in more complex configurations like M(cp)2(OTeF5)2 (M = Ti, Zr, Hf, Mo or W; cp = cyclopentadienyl, η5-C5H5)[21] or [AgOTeF5-(C6H5CH3)2]2.[17]
In the actinide series, uranyl compounds such as uranyl chloride (UO2Cl2) and [UO2Cl4]2− are well known and contain the linear UO2 moiety. Similar species exist for neptunium and plutonium.
Minerals and ionic compounds

Bismuth oxochloride (BiOCl, bismoclite) is a rare example of a mineral oxohalide. The crystal structure has a tetragonal symmetry and can be thought of as consisting of layers of Cl−, Bi3+ and O2− ions, in the order Cl-Bi-O-Bi-Cl-Cl-Bi-O-Bi-Cl. This layered, graphite-like structure results in a relatively low hardness of bismoclite (Mohs 2–2.5) and most other oxohalide minerals.[22] Those other minerals include terlinguaite Hg2OCl, formed by the weathering of mercury-containing minerals.[23] Mendipite, Pb3O2Cl2, formed from an original deposit of lead sulfide in a number of stages is another example of a secondary oxohalide mineral.
The elements iron, antimony, bismuth and lanthanum form oxochlorides of general formula MOCl. MOBr and MOI are also known for Sb and Bi. Many of their crystal structures have been determined.[24]
See also
References
- Synthesis of individual compounds can be found in Housecroft & Sharpe and Greenwood & Earnshaw in sections relating to the specific element, A
- Sisler, H. H. "Chromyl Chloride" Inorganic Synthesis McGraw-Hill: New York, 1946; Vol. 2, pp. 205–207.
- Greenwood & Earnshaw, p. 1023
- Greenwood & Earnshaw, p. 996.
- K. Nakamoto Infrared and Raman spectra of inorganic and coordination compounds, 5th. edition, Part A, Wiley, 1997 ISBN 0-471-19406-9, Tables II-4c, II-6g, II-6h, II-7b, II-8c
- Shriver & Atkins, Figure 13.8, p. 447
- Shriver & Atkins, p. 358
- Margrave, J. L.; Sharp, K. G.; Wilson, P. W. (9 September 1969). "Silicon-Fluorine Chemistry IX: The Reactions of Silicon Difluoride and Silicon Tetrafluoride with Water and Some Reactions of Tetrafluorodisiloxane". Journal of the American Chemical Society (published 25 March 1970). 92 (6). doi:10.1021/ja00709a015.
- Housecroft & Sharpe, pp. 329–330
- Housecroft & Sharpe, pp. 365–367
- Shriver & Atkins, p. 397
- Oberhammer, Heinz; Seppelt, Konrad (1978). "Molecular Structure of F5SOSF5, F5SeOSeF5, and F5TeOTeF5: d-Orbital Participation in Bonds between Main Group Elements". Angewandte Chemie International Edition. 17 (1): 69–70. doi:10.1002/anie.197800691.
- Housecroft & Sharpe, p. 395
- Fourati, Mohieddine; Chaabouni, Moncef; Belin, Claude Henri; Charbonnel, Monique; Pascal, Jean Louis; Potier, Jacqueline (1986). "A strongly chelating bidentate CLO4. New synthesis route and crystal structure determination of Ti(CLO4)". Inorg. Chem. 25 (9): 1386–1390. doi:10.1021/ic00229a019.
- Greenwood & Earnshaw, Chapters 22–25, section halides and oxohalides
- Greenwood & Earnshaw p. 993.
- Strauss, Steven H.; Noirot, Mark D.; Anderson, Oren P. (1985). "Preparation and characterization of silver(I) teflate complexes: bridging OTeF5 groups in the solid state and in solution". Inorg. Chem. 24 (25): 4307–4311. doi:10.1021/ic00219a022.
- Housectroft & Sharpe, Chapters 21 and 22 illustrate many structures, including M-O and M-Cl bond lengths.
- Dewan, John. C.; Edwards, Anthony J.; Calves, Jean Y.; Guerchais, Jacques E. (1997). "Fluoride crystal structures. Part 28. Bis(tetraethylammonium)μ-oxo-bis[pentafluorotantalate(V)]". J. Chem. Soc., Dalton Trans. (10): 978–980. doi:10.1039/DT9770000978.
{{cite journal}}: CS1 maint: multiple names: authors list (link). The structure is illustrated in Housectroft & Sharpe, Figure 22.5. - Housectroft & Sharpe, Figure 22.15.
- Crossman, Martin C.; Hope, Eric G.; Saunders, Graham C. (1996). "Cyclopentadienyl metal teflate (OTeF5) complexes". J. Chem. Soc., Dalton Trans. (4): 509–511. doi:10.1039/DT9960000509.
- Anthony, John W.; Bideaux, Richard A.; Bladh, Kenneth W.; Nichols, Monte C. (eds.). "Bismoclite". Handbook of Mineralogy (PDF). Vol. III (Halides, Hydroxides, Oxides). Chantilly, VA: Mineralogical Society of America. ISBN 0-9622097-2-4. Retrieved December 5, 2011.
- Hillebrand, W. F.; W. T. Schaller (1907). "Art. XXVI. The Mercury Minerals from Terlingua, Texas: Kleinite, Terlinguaite, Eglestonite, Montroydite, Calomel, Mercury". The American Journal of Science. s4-24 (139): 259–274. doi:10.2475/ajs.s4-24.141.259. Retrieved 2009-05-21.
- Wells, pp. 390–392
Bibliography
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Housecroft, C. E. and Sharpe, A. G. Inorganic Chemistry, 2nd ed., Pearson Prentice-Hall 2005. ISBN 0-582-31080-6
- Shrivr, D. F. and Atkins, P. W. Inorganic Chemistry, 3rd edn. Oxford University Press, 1999. ISBN 0-19-850330-X
- Wells, A. F. (1962). Structural Inorganic Chemistry (3rd ed.). Oxford: Clarendon Press. pp. 384–392. ISBN 0-19-855125-8..