PLAT domain
In molecular biology the PLAT domain is a protein domain that is found in a variety of membrane or lipid associated proteins. It is called the PLAT (Polycystin-1, Lipoxygenase, Alpha-Toxin) domain[2] or LH2 (Lipoxygenase homology) domain.[3] The known structure of pancreatic lipase shows this domain binds to procolipase Pfam PF01114, which mediates membrane association.
| PLAT/LH2 domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
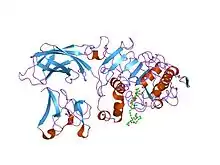 Structure of the lipase-procolipase complex.[1] | |||||||||
| Identifiers | |||||||||
| Symbol | PLAT | ||||||||
| Pfam | PF01477 | ||||||||
| InterPro | IPR001024 | ||||||||
| SMART | SM00308 | ||||||||
| PROSITE | PDOC50095 | ||||||||
| SCOP2 | 1lpa / SCOPe / SUPFAM | ||||||||
| OPM superfamily | 221 | ||||||||
| OPM protein | 3swz | ||||||||
| CDD | cd00113 | ||||||||
| |||||||||
This domain forms a beta-sandwich composed of two β-sheets of four β-strands each.[2][4][5]
Human proteins containing this domain
ALOX12; ALOX12B; ALOX12P2; ALOX15; ALOX15B; ALOX5; ALOXE3; LIPC; LIPG; LOXHD1; LPL; PKD1; PKD1L1; PKD1L2; PKD1L3; PKDREJ; PNLIP; PNLIPRP1; PNLIPRP2; PNLIPRP3; RAB6IP1;
References
- van Tilbeurgh H, Egloff MP, Martinez C, Rugani N, Verger R, Cambillau C (April 1993). "Interfacial activation of the lipase-procolipase complex by mixed micelles revealed by X-ray crystallography". Nature. 362 (6423): 814–20. doi:10.1038/362814a0. PMID 8479519. S2CID 4305832.
- Bateman A, Sandford R (1999). "The PLAT domain: a new piece in the PKD1 puzzle". Curr. Biol. 9 (16): R588–90. doi:10.1016/S0960-9822(99)80380-7. PMID 10469604. S2CID 15018010.
- Ponting CP, Hofmann K, Bork P (August 1999). "A latrophilin/CL-1-like GPS domain in polycystin-1". Curr. Biol. 9 (16): R585–8. doi:10.1016/S0960-9822(99)80379-0. PMID 10469603. S2CID 17252179.
- Delrieu I, Waller CC, Mota MM, Grainger M, Langhorne J, Holder AA (2002). "PSLAP, a protein with multiple adhesive motifs, is expressed in Plasmodium falciparum gametocytes". Mol. Biochem. Parasitol. 121 (1): 11–20. doi:10.1016/S0166-6851(02)00016-6. PMID 11985859.
- Minor W, Tomchick DR, Phan P, Cymborowski M, Holman TR (2001). "Structural and functional characterization of second-coordination sphere mutants of soybean lipoxygenase-1". Biochemistry. 40 (25): 7509–7517. doi:10.1021/bi002893d. PMID 11412104.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.