Tauopathy
Tauopathy belongs to a class of neurodegenerative diseases involving the aggregation of tau protein into neurofibrillary or gliofibrillary tangles in the human brain. Tangles are formed by hyperphosphorylation of the microtubule protein known as tau, causing the protein to dissociate from microtubules and form insoluble aggregates.[1] (These aggregations are also called paired helical filaments.) The mechanism of tangle formation is not well understood, and whether tangles are a primary cause of Alzheimer's disease or play a peripheral role is unknown.
| Tauopathy | |
|---|---|
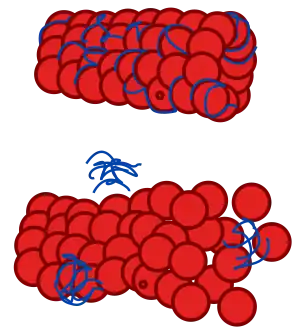 | |
| Diagram of a normal microtubule and one affected by tauopathy | |
| Specialty | Neurology |
Detection and imaging
- Post-mortem
- Tau tangles are seen microscopically in stained brain samples.[2]
Alzheimer's disease
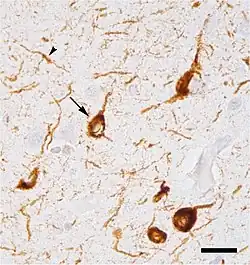
Neurofibrillary tangles were first described by Alois Alzheimer in one of his patients with Alzheimer's disease (AD). The tangles are considered a secondary tauopathy. AD is also classified as an amyloidosis because of the presence of senile plaques.[4]
When tau becomes hyperphosphorylated, the protein dissociates from the microtubules in axons.[5] Then, tau becomes misfolded and the protein begins to aggregate, which eventually forms the neurofibrillary tangles (NFT) seen in Alzheimer's patients.[1] Microtubules also destabilize when tau is dissociated. The combination of the neurofibrillary tangles and destabilized microtubules result in disruption of processes such as axonal transport and neural communication.[6]
The degree of NFT involvement in AD is defined by Braak stages. Braak stages I and II are used when NFT involvement is confined mainly to the transentorhinal region of the brain, stages III and IV when there is also involvement of limbic regions such as the hippocampus, and V and VI when there's extensive neocortical involvement. This should not be confused with the degree of senile plaque involvement, which progresses differently.[7]
Other diseases
- Primary age-related tauopathy (PART) dementia, with NFTs similar to AD, but without amyloid plaques.[4][8][9]
- Chronic traumatic encephalopathy (CTE)[10][11]
- Progressive supranuclear palsy (PSP)[12]
- Corticobasal degeneration (CBD)
- Frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17)[13]
- Vacuolar tauopathy[14]
- Lytico-bodig disease (Parkinson-dementia complex of Guam)[15]
- Ganglioglioma and gangliocytoma[16]
- Meningioangiomatosis[17]
- Postencephalitic parkinsonism
- Subacute sclerosing panencephalitis (SSPE)[18]
- As well as lead encephalopathy, tuberous sclerosis, pantothenate kinase-associated neurodegeneration, and lipofuscinosis[19]
In both Pick's disease and corticobasal degeneration, tau proteins are deposited as inclusion bodies within swollen or "ballooned" neurons.
Argyrophilic grain disease (AGD), another type of dementia,[20][21][22] is marked by an abundance of argyrophilic grains and coiled bodies upon microscopic examination of brain tissue.[23] Some consider it to be a type of Alzheimer's disease.[23] It may co-exist with other tauopathies such as progressive supranuclear palsy and corticobasal degeneration,[4] and also Pick's disease.[24]
Tauopathies are often overlapped with synucleinopathies, possibly due to interaction between the synuclein and tau proteins.[25]
The non-Alzheimer's tauopathies are sometimes grouped together as "Pick's complex" due to their association with frontotemporal dementia, or frontotemporal lobar degeneration.[26]
Research
It is found that activation of cannabinoid receptor type 1 (CB1) mediate inhibition of astroglial-derived nitric oxide (NO), that could be used as a new potential target to blunt tau protein hyperphosphorylation and the consequent related tauopathy in Alzheimer disease (AD).[27]
See also
References
- Goedert M, Spillantini MG (May 2017). "Propagation of Tau aggregates". Molecular Brain. 10 (1): 18. doi:10.1186/s13041-017-0298-7. PMC 5450399. PMID 28558799.
- Moloney C, et al. (May 2022). "Phosphorylated tau sites that are elevated in Alzheimer's disease fluid biomarkers are visualized in early neurofibrillary tangle maturity levels in the post mortem brain". Alzheimer's & Dementia.
- Alzheimer 'tau' protein far surpasses amyloid in predicting toll on brain tissue
- Dickson DW (August 2009). "Neuropathology of non-Alzheimer degenerative disorders". International Journal of Clinical and Experimental Pathology. 3 (1): 1–23. PMC 2776269. PMID 19918325.
- Wang JZ, Xia YY, Grundke-Iqbal I, Iqbal K (2013). "Abnormal hyperphosphorylation of tau: sites, regulation, and molecular mechanism of neurofibrillary degeneration". Journal of Alzheimer's Disease. 33 Suppl 1: S123-39. doi:10.3233/JAD-2012-129031. PMID 22710920.
- Wang Y, Mandelkow E (January 2016). "Tau in physiology and pathology". Nature Reviews. Neuroscience. 17 (1): 5–21. doi:10.1038/nrn.2015.1. PMID 26631930. S2CID 30614958.
- Braak H, Braak E (1991). "Neuropathological stageing of Alzheimer-related changes". Acta Neuropathologica. 82 (4): 239–59. doi:10.1007/BF00308809. PMID 1759558. S2CID 668690.
- Santa-Maria I, Haggiagi A, Liu X, Wasserscheid J, Nelson PT, Dewar K, Clark LN, Crary JF (November 2012). "The MAPT H1 haplotype is associated with tangle-predominant dementia". Acta Neuropathologica. 124 (5): 693–704. doi:10.1007/s00401-012-1017-1. PMC 3608475. PMID 22802095.
- Jellinger KA, Attems J (February 2007). "Neurofibrillary tangle-predominant dementia: comparison with classical Alzheimer disease". Acta Neuropathologica. 113 (2): 107–17. doi:10.1007/s00401-006-0156-7. PMID 17089134. S2CID 5655388.
- McKee AC, Cairns NJ, Dickson DW, Folkerth RD, Keene CD, Litvan I, Perl DP, Stein TD, Vonsattel JP, Stewart W, Tripodis Y, Crary JF, Bieniek KF, Dams-O'Connor K, Alvarez VE, Gordon WA (January 2016). "The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy" (PDF). Acta Neuropathologica. 131 (1): 75–86. doi:10.1007/s00401-015-1515-z. PMC 4698281. PMID 26667418.
- Roberts GW (1988). "Immunocytochemistry of neurofibrillary tangles in dementia pugilistica and Alzheimer's disease: evidence for common genesis". Lancet. 2 (8626–8627): 1456–8. doi:10.1016/S0140-6736(88)90934-8. PMID 2904573. S2CID 32662671.
- Williams DR, Lees AJ (March 2009). "Progressive supranuclear palsy: clinicopathological concepts and diagnostic challenges". The Lancet. Neurology. 8 (3): 270–9. doi:10.1016/S1474-4422(09)70042-0. PMID 19233037. S2CID 1417930.
- Selkoe DJ, Podlisny MB (2002). "Deciphering the genetic basis of Alzheimer's disease". Annual Review of Genomics and Human Genetics. 3: 67–99. doi:10.1146/annurev.genom.3.022502.103022. PMID 12142353.
- Darwich NF, Phan JM, et al. (2020). "Autosomal dominant VCP hypomorph mutation impairs disaggregation of PHF-tau". Science. 370 (6519): eaay8826. doi:10.1126/science.aay8826. PMC 7818661. PMID 33004675.
- Hof PR, Nimchinsky EA, Buée-Scherrer V, Buée L, Nasrallah J, Hottinger AF, Purohit DP, Loerzel AJ, Steele JC, Delacourte A (1994). "Amyotrophic lateral sclerosis/parkinsonism-dementia complex of Guam: quantitative neuropathology, immunohistochemical analysis of neuronal vulnerability, and comparison with related neurodegenerative disorders". Acta Neuropathologica. 88 (5): 397–404. doi:10.1007/BF00389490. PMID 7847067. S2CID 2821768.
- Brat DJ, Gearing M, Goldthwaite PT, Wainer BH, Burger PC (June 2001). "Tau-associated neuropathology in ganglion cell tumours increases with patient age but appears unrelated to ApoE genotype". Neuropathology and Applied Neurobiology. 27 (3): 197–205. doi:10.1046/j.1365-2990.2001.00311.x. PMID 11489139. S2CID 36482221.
- Halper J, Scheithauer BW, Okazaki H, Laws ER (July 1986). "Meningio-angiomatosis: a report of six cases with special reference to the occurrence of neurofibrillary tangles". Journal of Neuropathology and Experimental Neurology. 45 (4): 426–46. doi:10.1097/00005072-198607000-00005. PMID 3088216. S2CID 663552.
- Paula-Barbosa MM, Brito R, Silva CA, Faria R, Cruz C (November 1979). "Neurofibrillary changes in the cerebral cortex of a patient with subacute sclerosing panencephalitis (SSPE)". Acta Neuropathologica. 48 (2): 157–60. doi:10.1007/BF00691159. PMID 506699. S2CID 36105401.
- Wisniewski K, Jervis GA, Moretz RC, Wisniewski HM (March 1979). "Alzheimer neurofibrillary tangles in diseases other than senile and presenile dementia". Annals of Neurology. 5 (3): 288–94. doi:10.1002/ana.410050311. PMID 156000. S2CID 25649751.
- Ferrer I, Santpere G, van Leeuwen FW (June 2008). "Argyrophilic grain disease". Brain. 131 (Pt 6): 1416–32. doi:10.1093/brain/awm305. PMID 18234698.
- Josephs KA, Whitwell JL, Parisi JE, Knopman DS, Boeve BF, Geda YE, Jack CR, Petersen RC, Dickson DW (April 2008). "Argyrophilic grains: a distinct disease or an additive pathology?". Neurobiology of Aging. 29 (4): 566–73. doi:10.1016/j.neurobiolaging.2006.10.032. PMC 2727715. PMID 17188783.
- Wallon D, Sommervogel C, Laquerrière A, Martinaud O, Lecourtois M, Hannequin D (April 2010). "[Argyrophilic grain disease: synergistic component of dementia?]" [Argyrophilic grain disease: synergistic component of dementia?]. Revue Neurologique (in French). 166 (4): 428–32. doi:10.1016/j.neurol.2009.10.012. PMID 19963233.
- Tolnay M, Monsch AU, Staehelin HB, Probst A (May 1999). "[Argyrophilic grain disease: differentiation from Alzheimer disease]". Der Pathologe. 20 (3): 159–68. doi:10.1007/s002920050339. PMID 10412175. S2CID 2026154.
- Jellinger KA (April 1998). "Dementia with grains (argyrophilic grain disease)". Brain Pathology. 8 (2): 377–86. doi:10.1111/j.1750-3639.1998.tb00161.x. PMC 8098394. PMID 9546294. S2CID 22872309.
- Moussaud S, Jones DR, Moussaud-Lamodière EL, Delenclos M, Ross OA, McLean PJ (October 2014). "Alpha-synuclein and tau: teammates in neurodegeneration?". Molecular Neurodegeneration. 9: 43. doi:10.1186/1750-1326-9-43. PMC 4230508. PMID 25352339.
- Kertesz A, McMonagle P, Jesso S (November 2011). "Extrapyramidal syndromes in frontotemporal degeneration". Journal of Molecular Neuroscience. 45 (3): 336–42. doi:10.1007/s12031-011-9616-1. PMID 21887521. S2CID 13315112.
- Esposito, Giuseppe; De Filippis, Daniele; Steardo, Luca; Scuderi, Caterina; Savani, Claudia; Cuomo, Vincenzo; Iuvone, Teresa (2006-09-01). "CB1 receptor selective activation inhibits β-amyloid-induced iNOS protein expression in C6 cells and subsequently blunts tau protein hyperphosphorylation in co-cultured neurons". Neuroscience Letters. 404 (3): 342–346. doi:10.1016/j.neulet.2006.06.012. ISSN 0304-3940.