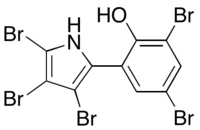
Pentabromopseudilin
Pentabromopseudilin, the first reported marine microbial antibiotic, is a bioactive natural product that contains a highly halogenated 2-arrylpyrrole moiety. Pentabromopseudilin (PBP) is a unique hybrid bromophenol-bromopyrrole compound that is made up of over 70% bromine atoms, contributing to its potent bioactivity. PBP was first isolated from Pseudomonas bromoutilis, and has since been found to be produced by other marine microbes, including Alteromonas luteoviolaceus, Chromobacteria, and Pseudoalteromonas spp.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2,4-Dibromo-6-(3,4,5-tribromo-1H-pyrrol-2-yl)phenol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H4Br5NO | |
| Molar mass | 553.668 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
History
PBP was first isolated and reported in 1966, when Burkholder and colleagues identified an unusual, highly brominated pyrrole antibiotic that was produced from Thalassia-associated marine bacteria collected from tropical waters off La Parguera, Puerto Rico.[1] Early investigations from this first report pointed at intrigue in regards to the pyrrole's particularly potent activity against gram positive bacteria, namely the strains Staphylococcus aureus, Diplococcus pneumoniae and Streptococcus pyogenes. However, despite inhibition these strains at PBP drug concentrations of 0.0063 μg/ml, these promising preliminary findings failed to yield the same success in in vivo mouse therapeutic studies.
Biological activity
Of more than 20 classified pyrrole antibiotics, PBP is the most active member in its class, and demonstrates a high in vitro activity (IC50 = 0.1 μM) against methicillin-resistant Staphylococcus aureus (MRSA).[2] As such, it has a served as a model example of the potential significance of marine natural products drug discovery as an effective resource in the face of "superbug" antibiotic resistance.[3][4] Other than being a potent antibiotic, PBP also demonstrates a wide variety of in vitro biological functions including antitumor activity, antifungal activity, myosin inhibition,[5] human lipoxygenase inhibition,[6] and phytotoxicity.[7]
Biosynthesis
The biosynthesis of PBP was first shown through isotope feeding studies, and then biochemically when genomic data of PBP-producing strains became readily available.
Early isotope feeding studies
The first investigations seeking to understand PBP biosynthesis was targeted by identifying PBP building blocks by feeding isotopically-labeled putative precursors of the phenol and pyrrole rings to PBP-producing bacteria. Identification of isotope labels being incorporated in the natural product would shed light on biosynthetic steps preceding the formation of PBP.
In 1994, a feeding experiment study conducted on Alteromonas luteoviolaceus demonstrated that the phenol ring of PBP is derived from the shikimate pathway.[8] In this work, general precursors that were labeled with 13C were fed to A. luteoviolaceus cultures after inoculation. It was found that the incorporation of a variety of glucose labels ([2-13C]-, [1,2-13C2]-, and [3-13C]), indicated that the PBP benzene ring is derived from carbohydrate metabolism and have occurred through erythrose 4-phosphate. From these results indicating a shikimic acid derivation, it was further deduced that p-hydroxybenzoic acid (4-HBA) be a plausible precursor for PBP's phenol group.[9] This was indeed proven when isotopically labeled 4-HBA was observed to be incorporated into the phenol ring of PBP at >80%.[10] Despite this groundbreaking study, no 13C incorporation was observed in the pyrrole ring of PBP.
In 2004, a follow-up study described the PBP pyrrole ring as derived from L-proline.[11] This work involved an isotope-feeding study involving 21 different Alteromonas luteoviolaceus culture medias that were introduced with labeled [5-13C]proline in addition to tyrosine, histidine, ornithine, glycine, potassium bromide (KBr), and PBP. From this feeding experiment, it was determined that the overall enrichment of PBP had increased by 60%- indicating that proline had directly converted into the pyrrole ring.
Genetic basis for pentabromopseudilin biosynthesis
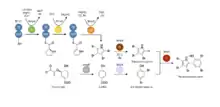
The biosynthesis of pentabromopseudilin, among other halopyrroles, has been elucidated via the identification of the conserved brominated marine pyrroles/phenols (bmp) pathway first identified in the marine bacteria, P. luteoviolacea 2ta16 and P. phenolica O-BC30.[12] The bmp pathway describes a bi-modular scheme for polybrominated marine microbial natural products production. In the first module, the bromophenol moiety of PBP is first assembled from a chorismate precursor which is then converted by Bmp6, a chorismate lyase (CL), to yield 4-HBA. 4-HBA is then di-halogenated by the flavin-dependent halogenase, Bmp5 (Hal), to yield 2,3-dibromophenol.
Consistent with earlier isotopic studies, the PBP halopyrrole biosynthesis via the bmp pathway begins with L-proline. In this module, L-proline is acylated to the acyl carrier protein domain of Bmp1 (ACP-thioesterase (TE) di-domain protein) by the proline adenyl transferase Bmp4 (A). The flavin-dependent dehydrogenase, Bmp3 (DH), then oxidizes the prolyl ring to a pyrrole.[13] The loaded protein is then tri-brominated with the flavin-dependent halogenase, Bmp2 (Hal), becoming 2,3,4,5-tetrabromopyrrole. Next, Bmp8 (D), a unique thioredoxin-like dehalogenase, removes the C-2 bromine of tetrabromopyrrole,[14] which then allows for coupling to the previously described 2,4-dibromophenol via the cytochrome P450 enzyme, Bmp7 (C).
References
- Burkholder, Paul R.; Pfister, Robert M.; Leitz, Frederick H. (1 July 1966). "Production of a Pyrrole Antibiotic by a Marine Bacterium1". Applied Microbiology. 14 (4): 649–653. doi:10.1128/AM.14.4.649-653.1966. ISSN 0003-6919. PMC 546803. PMID 4380876.
- Schwalm, Cristiane S.; de Castro, Ilton B.D.; Ferrari, Jailton; de Oliveira, Fábio L.; Aparicio, Ricardo; Correia, Carlos Roque D. (March 2012). "Synthesis of pentabromopseudilin and other arylpyrrole derivatives via Heck arylations". Tetrahedron Letters. 53 (13): 1660–1663. doi:10.1016/j.tetlet.2012.01.086.
- LAATSCH, Hartmut; RENNEBERG, Bernd; HANEFELD, Ulf; KELLNER, Michael; PUDLEINER, Heinz; HAMPRECHT, Gerhard; KRAEMER, Hans-Peter; ANKE, Heidrun (1995). "Structure-Activity Relationships of Phenyl- and Benzoylpyrroles". Chemical & Pharmaceutical Bulletin. 43 (4): 537–546. doi:10.1248/cpb.43.537. PMID 7600609.
- Rahman, Hafizur; Austin, Brian; Mitchell, Wilfrid J.; Morris, Peter C.; Jamieson, Derek J.; Adams, David R.; Spragg, Andrew Mearns; Schweizer, Michael (5 March 2010). "Novel Anti-Infective Compounds from Marine Bacteria". Marine Drugs. 8 (3): 498–518. doi:10.3390/md8030498. PMC 2857357. PMID 20411112.
- Fedorov, Roman; Böhl, Markus; Tsiavaliaris, Georgios; Hartmann, Falk K; Taft, Manuel H; Baruch, Petra; Brenner, Bernhard; Martin, René; Knölker, Hans-Joachim; Gutzeit, Herwig O; Manstein, Dietmar J (4 January 2009). "The mechanism of pentabromopseudilin inhibition of myosin motor activity". Nature Structural & Molecular Biology. 16 (1): 80–88. doi:10.1038/nsmb.1542. PMID 19122661. S2CID 17109386.
- Ohri, Rachana V.; Radosevich, Alexander T.; Hrovat, K. James; Musich, Christine; Huang, David; Holman, Theodore R.; Toste, F. Dean (June 2005). "A Re(V)-Catalyzed C−N Bond-Forming Route to Human Lipoxygenase Inhibitors". Organic Letters. 7 (12): 2501–2504. doi:10.1021/ol050897a. PMID 15932233.
- Schwalm, Cristiane S.; de Castro, Ilton B.D.; Ferrari, Jailton; de Oliveira, Fábio L.; Aparicio, Ricardo; Correia, Carlos Roque D. (March 2012). "Synthesis of pentabromopseudilin and other arylpyrrole derivatives via Heck arylations". Tetrahedron Letters. 53 (13): 1660–1663. doi:10.1016/j.tetlet.2012.01.086.
- Hanefeld, Ulf; Floss, Heinz G.; Laatsch, Hartmut (July 1994). "Biosynthesis of the marine antibiotic pentabromopseudilin. Part 1. The benzene ring". The Journal of Organic Chemistry. 59 (13): 3604–3608. doi:10.1021/jo00092a020.
- Atta-ur-Rahman, edited by (2000). Bioactive natural products (1. ed.). Amsterdam: Elsevier. ISBN 978-0-444-50469-2.
{{cite book}}:|first1=has generic name (help) - Hanefeld, Ulf; Floss, Heinz G.; Laatsch, Hartmut (July 1994). "Biosynthesis of the marine antibiotic pentabromopseudilin. Part 1. The benzene ring". The Journal of Organic Chemistry. 59 (13): 3604–3608. doi:10.1021/jo00092a020.
- PESCHKE, Jörg D.; HANEFELD, Ulf; LAATSCH, Hartmut (22 May 2014). "Biosynthesis of the Marine Antibiotic Pentabromopseudilin. 2. The Pyrrole Ring". Bioscience, Biotechnology, and Biochemistry. 69 (3): 628–630. doi:10.1271/bbb.69.628. PMID 15784994.
- Agarwal, Vinayak; El Gamal, Abrahim A; Yamanaka, Kazuya; Poth, Dennis; Kersten, Roland D; Schorn, Michelle; Allen, Eric E; Moore, Bradley S (29 June 2014). "Biosynthesis of polybrominated aromatic organic compounds by marine bacteria". Nature Chemical Biology. 10 (8): 640–647. doi:10.1038/NCHEMBIO.1564. PMC 4104138. PMID 24974229.
- El Gamal, Abrahim; Agarwal, Vinayak; Diethelm, Stefan; Rahman, Imran; Schorn, Michelle A.; Sneed, Jennifer M.; Louie, Gordon V.; Whalen, Kristen E.; Mincer, Tracy J.; Noel, Joseph P.; Paul, Valerie J.; Moore, Bradley S. (5 April 2016). "Biosynthesis of coral settlement cue tetrabromopyrrole in marine bacteria by a uniquely adapted brominase–thioesterase enzyme pair". Proceedings of the National Academy of Sciences. 113 (14): 3797–3802. Bibcode:2016PNAS..113.3797E. doi:10.1073/pnas.1519695113. PMC 4833250. PMID 27001835.
- El Gamal, Abrahim; Agarwal, Vinayak; Rahman, Imran; Moore, Bradley S. (12 October 2016). "Enzymatic Reductive Dehalogenation Controls the Biosynthesis of Marine Bacterial Pyrroles". Journal of the American Chemical Society. 138 (40): 13167–13170. doi:10.1021/jacs.6b08512. PMC 5201201. PMID 27676265.