Pentazolate
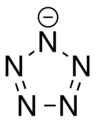
In chemistry, a pentazolate is a compound that contains a cyclo-N5− ion, the anion of pentazole. In 2017, researchers prepared the first salt (N5)6(H3O)3(NH4)4Cl containing pentazolate anion starting a substituted phenylpentazole, m-CPBA and iron(II) glycinate.[1] A series of metal and nonmetal pentazolates were subsequently synthesized according to their work.
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
| Properties | |
| N5−1 | |
| Molar mass | 70.036 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
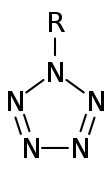
Precursors to pentazolates. (R=3,5-dimethyl-4-hydroxylphenyl)
List of pentazolates
| Appearance | Dec. temp (°C) | Ref | |
|---|---|---|---|
| LiN5 | white | 133 | [2] |
| LiN5·3H2O | colorless | 139 | [2] |
| [Na(N5)(H2O)]·2H2O | colorless | 111.3 | [3] |
| [Mg(N5)2(H2O)6]·4H2O | colorless | 103.5 | [3] |
| Al(H2O)6(N5)3·9H2O | white | 141.4 | [4] |
| [Mn(N5)2(H2O)4]·4H2O | colorless | 104.1 | [3] |
| [Fe(N5)2(H2O)4]·4H2O | pale green | 114.7 | [3] |
| Fe(H2O)6(N5)3·9H2O | reddish brown | 108.9 | [4] |
| [Co(N5)2(H2O)4]·4H2O | bright orange | 58.9 | [3] |
| AgN5 | [5] | ||
| [Pb(OH)]4(N5)4 | colorless | 80 | [6] |
| [N(CH3)4]N5 | white | 80.8 | [7] |
| (NH3OH)N5 | white | 104.3 | [7] |
| [C(NH2)3]N5 | white | 88.1 | [7] |
| NH4N5 | white | 102.0 | [7] |
| N2H5N5 | white | 85.3 | [7] |
| (CN7H6)N5 | 96.3 | [8] |
References
- Zhang, C., Sun, C., Hu, B., Yu, C., & Lu, M. (2017). Synthesis and characterization of the pentazolate anion cyclo-N5− in (N5)6(H3O)3(NH4)4Cl. Science, 355(6323), 374–376. doi:10.1126/science.aah3840
- Xu, Y., Ding, L., Yang, F., Li, D., Wang, P., Lin, Q., & Lu, M. (2022). LiN5: A novel pentazolate salt with high nitrogen content. Chemical Engineering Journal, 429, 132399.
- Xu, Y., Wang, Q., Shen, C. et al. A series of energetic metal pentazolate hydrates. Nature 549, 78–81 (2017). doi:10.1038/nature23662
- Chen, L., Yang, C., Hu, H., Shi, L., Zhang, C., Sun, C., ... & Hu, B. (2022). Iron(III) and aluminum(III) complexes of cyclopentazolate anions. CrystEngComm. doi:10.1039/D2CE01270G
- Sun, C., Zhang, C., Jiang, C., Yang, C., Du, Y., Zhao, Y., Hu. B., Zheng. Z., Christe, K. O. (2018). Synthesis of AgN5 and its extended 3D energetic framework. Nature Communications, 9(1). doi:10.1038/s41467-018-03678-y
- Yuan, Y., Xu, Y., Xie, Q., Li, D., Lin, Q., Wang, P., & Lu, M. (2022). Pentazolate coordination polymers self-assembled by in situ generated [Pb4(OH)4]4+ cubic cations trapping cyclo-N5–. Dalton Transactions, 51(15), 5801-5809.
- Yang, C., Zhang, C., Zheng, Z., Jiang, C., Luo, J., Du, Y., Hu. B., Sun. C., Christe, K. O. (2018). Synthesis and Characterization of cyclo-Pentazolate Salts of NH4+, NH3OH+, N2H5+, C(NH2)3+, and N(CH3)4+. Journal of the American Chemical Society. doi:10.1021/jacs.8b05106
- Yu, R. J., Liu, Y. J., Huang, W., & Tang, Y. X. (2022). A hybrid of tetrazolium and pentazolate: An energetic salt with ultrahigh nitrogen content and energy. Energetic Materials Frontiers. doi:10.1016/j.enmf.2022.05.002
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.