Petrogenetic grid
A petrogenetic grid is a geological phase diagram that connects the stability ranges or metastability ranges of metamorphic minerals or mineral assemblages to the conditions of metamorphism. Experimentally determined mineral or mineral-assemblage stability ranges are plotted as metamorphic reaction boundaries in a pressure–temperature cartesian coordinate system to produce a petrogenetic grid for a particular rock composition. The regions of overlap of the stability fields of minerals form equilibrium mineral assemblages used to determine the pressure–temperature conditions of metamorphism. This is particularly useful in geothermobarometry.[3][4][5][6]
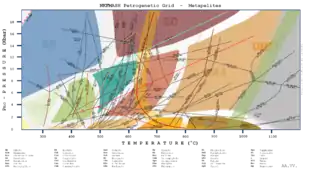
Figure 1 is an example of a complex petrogenetic grid for metamorphosed pelitic rocks. It shows most of the important reactions that govern the development of aluminous mineral assemblages from the prehnite-pumpellyite facies to the granulite facies, as well as the blueschist facies and eclogite facies at higher pressures and the contact hornfels facies at lower pressures. As the rock undergoes higher temperatures and pressures, it follows the classic Barrovian sequence from the chlorite zone to the biotite zone to the garnet zone to the staurolite zone.
For a metapelitic rock containing chlorite, kaolinite, and quartz the petrogenetic grid for metapelites (Figure 1) shows that such a rock can only form at relatively low pressures and temperatures. However, if it had carpholite instead of chlorite, then it would have formed at higher pressures, and if it had pyrophyllite instead of kaolinite, it would have formed at higher temperatures. This assumes the rock has a KFMASH (K2O–FeO–MgO–Al2O3–SiO2–H2O) composition because that is what the experimental data was created with. If the composition of the rock differs from this, then the figure is less accurate.
Norman L. Bowen proposed the concept of petrogenetic grids in 1940.[7] At the time, he envisioned geologists eventually determining every possible metamorphic reaction and assemblage in nature, but realized that the magnitude of undertaking the necessary experiments was a huge task that would not be finished for a very long time. As such, modern petrogenetic grids are only partially complete. Depending on the level of precision and characterization needed, a petrogenetic grid may be simple, or it may be an extremely large system consisting of a hundred or more reactions.
See also
- Compatibility diagram – Depicts coexisting mineral phases in a metamorphic rock
- Metamorphic rock – Rock that was subjected to heat and pressure
References
- Wei, Chunjing; Powell, Roger (2003). "Phase relations in high-pressure metapelites in the system KFMASH (K2O–FeO–MgO–Al2O3–SiO2–H2O) with application to natural rocks". Contributions to Mineralogy and Petrology. 145 (3): 301–315. doi:10.1007/s00410-003-0454-1.
- Wei, Chunjing; Powell, Roger; Clarke, Gordon (2004). "Calculated phase equilibria for low‐ and medium‐pressure metapelites in the KFMASH and KMnFMASH systems". Journal of Metamorphic Geology. 22 (5): 495–508. doi:10.1111/j.1525-1314.2004.00530.x.
- Proyer, A (2003). "Metamorphism of pelites in NKFMASH — A new petrogenetic grid with implications for the preservation of high-pressure mineral assemblages during exhumation". Journal of Metamorphic Geology. 22 (5): 493–509. doi:10.1046/j.1525-1314.2003.00457.x.
- Spear, Frank; Cheney, John (1989). "A petrogenetic grid for pelitic schists in the system SiO2-Al2O3-FeO-MgO-K2O-H2O". Contributions to Mineralogy and Petrology. 101 (2): 149–164. doi:10.1007/BF00375302.
- Carrington, D; Harley, S (1995). "Partial melting and phase relations in high-grade metapelites: an experimental petrogenetic grid in the KFMASH system". Contributions to Mineralogy and Petrology. 120 (3–4): 270–291. doi:10.1007/BF00306508.
- Pattison, David; Spear, Frank (2018). "Kinetic control of staurolite–Al2SiO5 mineral assemblages: Implications for Barrovian and Buchan metamorphism". Journal of Metamorphic Geology. 36 (6): 667–690. doi:10.1111/jmg.12302.
- Bowen, Norman (1940). "Progressive Metamorphism of Siliceous Limestone and Dolomite". The Journal of Geology. 48 (3): 225–274. doi:10.1086/624885.
- Whitney, D.L. (2002). "Coexisting andalusite, kyanite, and sillimanite: Sequential formation of three Al2SiO5 polymorphs during progressive metamorphism near the triple point, Sivrihisar, Turkey". American Mineralogist. 87 (4): 405–416. doi:10.2138/am-2002-0404.
- Whitney, D.L. (2002). "Coexisting andalusite, kyanite, and sillimanite: Sequential formation of three Al2SiO5 polymorphs during progressive metamorphism near the triple point, Sivrihisar, Turkey". American Mineralogist. 87 (4): 405–416. doi:10.2138/am-2002-0404.
Further reading
Winter, John (2013). Principles of Igneous and Metamorphic Petrology. Pearson Education Limited. ISBN 978-0321592576.
