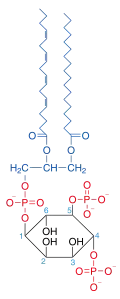
Phosphatidylinositol 4,5-bisphosphate
Phosphatidylinositol 4,5-bisphosphate or PtdIns(4,5)P2, also known simply as PIP2 or PI(4,5)P2, is a minor phospholipid component of cell membranes. PtdIns(4,5)P2 is enriched at the plasma membrane where it is a substrate for a number of important signaling proteins.[1] PIP2 also forms lipid clusters[2] that sort proteins.[3][4][5]
 | |
| Names | |
|---|---|
| IUPAC name
1,2-Diacyl-sn-glycero-3-phospho-(1-D-myo-inositol 4,5-bisphosphate) | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C47H80O19P3 | |
| Molar mass | 1042.05 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
PIP2 is formed primarily by the type I phosphatidylinositol 4-phosphate 5-kinases from PI(4)P. In metazoans, PIP2 can also be formed by type II phosphatidylinositol 5-phosphate 4-kinases from PI(5)P.[6]
The fatty acids of PIP2 are variable in different species and tissues, but the most common fatty acids are stearic in position 1 and arachidonic in 2.[7]
Signaling pathways
PIP2 is a part of many cellular signaling pathways, including PIP2 cycle, PI3K signalling, and PI5P metabolism.[8] Recently, it has been found in the nucleus[9] with unknown function.
Functions
Cytoskeleton dynamics near membranes
PIP2 regulates the organization, polymerization, and branching of filamentous actin (F-actin) via direct binding to F-actin regulatory proteins.[10]
Endocytosis and exocytosis
The first evidence that indicated phosphoinositides(PIs) (especially PI(4,5)P2) are important during the exocytosis process was in 1990. Emberhard et al. [11] found that the application of PI-specific phospholipase C into digitonin-permeabilized chromaffin cells decreased PI levels, and inhibited calcium-triggered exocytosis. This exocytosis inhibition was preferential for an ATP-dependent stage, indicating PI function was required for secretion. Later studies identified associated proteins necessary during this stage, such as phosphatidylinositol transfer protein ,[12] and phosphoinositol-4-monophosphatase 5 kinase type Iγ (PIPKγ) ,[13] which mediates PI(4,5)P2 restoration in permeable cell incubation in an ATP-dependent way. In these later studies, PI(4,5)P2 specific antibodies strongly inhibited exocytosis, thus providing direct evidence that PI(4,5)P2 plays a pivotal role during the LDCV (Large dense core vesicle) exocytosis process.
Through the use of PI-specific kinase/phosphatase identification and PI antibody/drug/blocker discovery, the role of PI (especially PI(4,5)P2) in secretion regulation was extensively investigated. Studies utilizing PHPLCδ1 domain over-expression (acting as PI(4,5)P2 buffer or blocker) ,[14] PIPKIγ knockout in chromaffin cell [15] and in central nerve system,[16] PIPKIγ knockdown in beta cell lines ,[17] and over-expression of membrane-tethered inositol 5-phosphatase domain of synaptojanin 1 ,[18] all suggested vesicle (synaptic vesicle and LDCV) secretion were severely impaired after PI(4,5)P2 depletion or blockage. Moreover, some studies[18][16][15] showed an impaired/reduced RRP of those vesicles, though the docked vesicle number were not altered[15] after PI(4,5)P2 depletion, indicating a defect at a pre-fusion stage (priming stage). Follow-up studies indicated that PI(4,5)P2 interactions with CAPS,[19] Munc13[20] and synaptotagmin1[21] are likely to play a role in this PI(4,5)P2 dependent priming defect.
IP3/DAG pathway
PIP2 functions as an intermediate in the IP3/DAG pathway, which is initiated by ligands binding to G protein-coupled receptors activating the Gq alpha subunit. PtdIns(4,5)P2 is a substrate for hydrolysis by phospholipase C (PLC), a membrane-bound enzyme activated through protein receptors such as α1 adrenergic receptors. PIP2 regulates the function of many membrane proteins and ion channels, such as the M-channel. The products of the PLC catalyzation of PIP2 are inositol 1,4,5-trisphosphate (InsP3; IP3) and diacylglycerol (DAG), both of which function as second messengers. In this cascade, DAG remains on the cell membrane and activates the signal cascade by activating protein kinase C (PKC). PKC in turn activates other cytosolic proteins by phosphorylating them. The effect of PKC could be reversed by phosphatases. IP3 enters the cytoplasm and activates IP3 receptors on the smooth endoplasmic reticulum (ER), which opens calcium channels on the smooth ER, allowing mobilization of calcium ions through specific Ca2+ channels into the cytosol. Calcium participates in the cascade by activating other proteins.[22]
Docking phospholipids
Class I PI 3-kinases phosphorylate PtdIns(4,5)P2 forming phosphatidylinositol (3,4,5)-trisphosphate (PtdIns(3,4,5)P3) and PtdIns(4,5)P2 can be converted from PtdIns4P. PtdIns4P, PtdIns(3,4,5)P3 and PtdIns(4,5)P2 not only act as substrates for enzymes but also serve as docking phospholipids that bind specific domains that promote the recruitment of proteins to the plasma membrane and subsequent activation of signaling cascades.[23][24]
- Examples of proteins activated by PtdIns(3,4,5)P3 are Akt, PDPK1, Btk1.
- One mechanism for direct effect of PtdIns(4,5)P2 is opening of Na+ channels as a minor function in growth hormone release by growth hormone-releasing hormone.[25]
Potassium channels
Inwardly rectifying potassium channels have been shown to require docking of PIP2 for channel activity.[26][27]
G protein-coupled receptors
PtdIns(4,5)P2 has been shown to stabilize the active states of Class A G protein-coupled receptors (GPCRs) via direct binding, and enhance their selectivity toward certain G proteins.[28]
G protein-coupled receptor kinases
PIP2 has been shown to recruit G protein-coupled receptor kinase 2 (GRK2) to the membrane by binding to the large lobe of GRK2. This stabilizes GRK2 and also orients it in a way that allows for more efficient phosphorylation of the beta adrenergic receptor, a type of GPCR.[29]
Regulation
PIP2 is regulated by many different components. One emerging hypothesis is that PIP2 concentration is maintained locally. Some of the factors involved in PIP2 regulation are:[30]
- Lipid kinases, Lipid Phosphatase
- Lipid Transfer Proteins
- Growth Factors, Small GTPases
- Cell Attachment
- Cell-Cell Interaction
- Change in cell volume
- Cell differentiation state
- Cell stress
References
- Strachan T, Read AP (1999). Leptospira. In: Human Molecular Genetics (2nd ed.). Wiley-Liss. ISBN 0-471-33061-2. (via NCBI Bookshelf).
- van den Bogaart, G; Meyenberg, K; Risselada, HJ; Amin, H; Willig, KI; Hubrich, BE; Dier, M; Hell, SW; Grubmüller, H; Diederichsen, U; Jahn, R (23 October 2011). "Membrane protein sequestering by ionic protein-lipid interactions". Nature. 479 (7374): 552–5. Bibcode:2011Natur.479..552V. doi:10.1038/nature10545. hdl:11858/00-001M-0000-0012-5C28-1. PMC 3409895. PMID 22020284. S2CID 298052.
- Petersen, EN; Chung, HW; Nayebosadri, A; Hansen, SB (15 December 2016). "Kinetic disruption of lipid rafts is a mechanosensor for phospholipase D." Nature Communications. 7: 13873. Bibcode:2016NatCo...713873P. doi:10.1038/ncomms13873. PMC 5171650. PMID 27976674. S2CID 14678865.
- Yuan, Z; Pavel, MA; Wang, H; Kwachukwu, JC; Mediouni, S; Jablonski, JA; Nettles, KW; Reddy, CB; Valente, ST; Hansen, SB (14 September 2022). "Hydroxychloroquine blocks SARS-CoV-2 entry into the endocytic pathway in mammalian cell culture". Communications Biology. 5 (1): 958. doi:10.1038/s42003-022-03841-8. PMC 9472185. PMID 36104427. S2CID 252281018.
- Robinson, CV; Rohacs, T; Hansen, SB (September 2019). "Tools for Understanding Nanoscale Lipid Regulation of Ion Channels". Trends in Biochemical Sciences. 44 (9): 795–806. doi:10.1016/j.tibs.2019.04.001. PMC 6729126. PMID 31060927. S2CID 146810646.
- Rameh, LE; Tolias, K; Duckworth, BC; Cantley, LC (Nov 1997). "A new pathway for synthesis of phosphatydilinositol-4,5-bisphosphate". Nature. 390 (6656): 192–6. doi:10.1038/36621. PMID 9367159. S2CID 4403301.
- Tanaka T, Iwawaki D, Sakamoto M, Takai Y, Morishige J, Murakami K, Satouchi K (April 2003). "Mechanisms of accumulation of arachidonate in phosphatidylinositol in yellowtail. A comparative study of acylation systems of phospholipids in rat and the fish species Seriola quinqueradiata". Eur J Biochem. 270 (7): 1466–73. doi:10.1046/j.1432-1033.2003.03512.x. PMID 12654002.
- Bulley SJ, Clarke JH, Droubi A, Giudici ML, Irvine RF (2015). "Exploring phosphatidylinositol 5-phosphate 4-kinase function". Adv Biol Regul. 57: 193–202. doi:10.1016/j.jbior.2014.09.007. PMC 4359101. PMID 25311266.
- Lewis AE, Sommer L, Arntzen MØ, Strahm Y, Morrice NA, Divecha N, D'Santos CS (2011). "Identification of nuclear phosphatidylinositol 4,5-bisphosphate-interacting proteins by neomycin extraction". Mol Cell Proteomics. 10 (2): M110.003376. doi:10.1074/mcp.M110.003376. PMC 3033679. PMID 21048195.
- Sun, Hui; Yamamoto, Masaya; Mejillano, Marisan; Yin, Helen (November 19, 1999). "Gelsolin, a Multifunctional Actin Regulatory Protein". The Journal of Biological Chemistry. 274 (47): 33179–82. doi:10.1074/jbc.274.47.33179. PMID 10559185.
- Eberhard, David A, et al. (1990). "Evidence that the inositol phospholipids are necessary for exocytosis. Loss of inositol phospholipids and inhibition of secretion in permeabilized cells caused by a bacterial phospholipase C and removal of ATP". Biochemical Journal. 268 (1): 15–25. doi:10.1042/bj2680015. PMC 1131385. PMID 2160809.
- Hay, Jesse C, Thomas M (1993). "Phosphatidylinositol transfer protein required for ATP-dependent priming of Ca2+-activated secretion". Nature. 366 (6455): 572–575. doi:10.1038/366572a0. PMID 8255295. S2CID 4348488.
- Hay, Jesse C, et al. (1995). "ATP-dependent inositide phosphorylation required for Ca2positive-activated secretion". Nature. 374 (6518): 173–177. doi:10.1038/374173a0. PMID 7877690. S2CID 4365980.
- Holz RW, et al. (2000). "A pleckstrin homology domain specific for phosphatidylinositol 4, 5-bisphosphate (PtdIns-4, 5-P2) and fused to green fluorescent protein identifies plasma membrane PtdIns-4, 5-P2 as being important in exocytosis". J. Biol. Chem. 275 (23): 17878–17885. doi:10.1074/jbc.M000925200. PMID 10747966.
- Gong LW, et al. (2005). "Phosphatidylinositol phosphate kinase type Iγ regulates dynamics of large dense-core vesicle fusion". PNAS. 102 (14): 5204–5209. doi:10.1073/pnas.0501412102. PMC 555604. PMID 15793002.
- Di Paolo G, et al. (2004). "Impaired PtdIns (4, 5) P2 synthesis in nerve terminals produces defects in synaptic vesicle trafficking". Nature. 431 (7007): 415–422. doi:10.1038/nature02896. PMID 15386003. S2CID 4333681.
- Waselle L, et al. (2005). "Role of phosphoinositide signaling in the control of insulin exocytosis". Molecular Endocrinology. 19 (12): 3097–3106. doi:10.1210/me.2004-0530. PMID 16081518.
- Milosevic I, et al. (2005). "Plasmalemmal phosphatidylinositol-4, 5-bisphosphate level regulates the releasable vesicle pool size in chromaffin cells". Journal of Neuroscience. 25 (10): 2557–2565. doi:10.1523/JNEUROSCI.3761-04.2005. PMC 6725155. PMID 15758165.
- Grishanin RN, et al. (2004). "CAPS acts at a prefusion step in dense-core vesicle exocytosis as a PIP 2 binding protein". Neuron. 43 (4): 551–562. doi:10.1016/j.neuron.2004.07.028. PMID 15312653.
- Kabachinski G, et al. (2014). "CAPS and Munc13 utilize distinct PIP2-linked mechanisms to promote vesicle exocytosis". Molecular Biology of the Cell. 25 (4): 508–521. doi:10.1091/mbc.E12-11-0829. PMC 3923642. PMID 24356451.
- Loewen CA, et al. (2006). "C2B polylysine motif of synaptotagmin facilitates a Ca2+-independent stage of synaptic vesicle priming in vivo". Molecular Biology of the Cell. 17 (12): 5211–5226. doi:10.1091/mbc.E06-07-0622. PMC 1679685. PMID 16987956.
- Rusten, Tor Erik; Stenmark, Harald (April 2006). "Analyzing phosphoinositides and their interacting proteins". Nature Methods. 3 (4): 251–258. doi:10.1038/nmeth867. ISSN 1548-7091. PMID 16554828. S2CID 20289175.
- Won DH, et al. (2006). "PI (3, 4, 5) P3 and PI (4, 5) P2 lipids target proteins with polybasic clusters to the plasma membrane". Science. 314 (5804): 1458–1461. doi:10.1126/science.1134389. PMC 3579512. PMID 17095657.
- Hammond G, et al. (2012). "PI4P and PI (4, 5) P2 are essential but independent lipid determinants of membrane identity". Science. 337 (6095): 727–730. doi:10.1126/science.1222483. PMC 3646512. PMID 22722250.
- GeneGlobe -> GHRH Signaling Retrieved on May 31, 2009
- Soom, M (2001). "Multiple PtdIns(4,5)P2 binding sites in Kir2.1 inwardly rectifying potassium channels". FEBS Letters. 490 (1–2): 49–53. doi:10.1016/S0014-5793(01)02136-6. PMID 11172809. S2CID 36375203.
- Hansen, SB; Tao, X; MacKinnon, R (28 August 2011). "Structural basis of PIP2 activation of the classical inward rectifier K+ channel Kir2.2". Nature. 477 (7365): 495–8. doi:10.1038/nature10370. PMC 3324908. PMID 21874019.
- Yen, Hsin-Yung; Hoi, Kin Kuan; Liko, Idlir; Hedger, George; Horrell, Michael R.; Song, Wanling; Wu, Di; Heine, Philipp; Warne, Tony (2018-07-11). "PtdIns(4,5)P2 stabilizes active states of GPCRs and enhances selectivity of G-protein coupling". Nature. 559 (7714): 423–427. doi:10.1038/s41586-018-0325-6. ISSN 0028-0836. PMC 6059376. PMID 29995853.
- Yang, Pei; Homan, Kristoff T.; Li, Yaoxin; Cruz-Rodríguez, Osvaldo; Tesmer, John J.G.; Chen, Zhan (2016-05-24). "Effect of Lipid Composition on Membrane Orientation of the G protein-coupled Receptor Kinase 2-Gβ1γ2 Complex". Biochemistry. 55 (20): 2841–2848. doi:10.1021/acs.biochem.6b00354. ISSN 0006-2960. PMC 4886744. PMID 27088923.
- Hilgemann, D. W. (2001). "The Complex and Intriguing Lives of PIP2 with Ion Channels and Transporters". Science's STKE. 2001 (111): 19re–19. doi:10.1126/stke.2001.111.re19. PMID 11734659. S2CID 24745275.