Photoplethysmogram
A photoplethysmogram (PPG) is an optically obtained plethysmogram that can be used to detect blood volume changes in the microvascular bed of tissue.[1][2] A PPG is often obtained by using a pulse oximeter which illuminates the skin and measures changes in light absorption.[3] A conventional pulse oximeter monitors the perfusion of blood to the dermis and subcutaneous tissue of the skin.

| Photoplethysmography | |
|---|---|
 Representative PPG taken from an ear pulse oximeter. Variation in amplitude are from Respiratory Induced Variation. | |
| MeSH | D017156 |
With each cardiac cycle the heart pumps blood to the periphery. Even though this pressure pulse is somewhat damped by the time it reaches the skin, it is enough to distend the arteries and arterioles in the subcutaneous tissue. If the pulse oximeter is attached without compressing the skin, a pressure pulse can also be seen from the venous plexus, as a small secondary peak.
The change in volume caused by the pressure pulse is detected by illuminating the skin with the light from a light-emitting diode (LED) and then measuring the amount of light either transmitted or reflected to a photodiode.[4] Each cardiac cycle appears as a peak, as seen in the figure. Because blood flow to the skin can be modulated by multiple other physiological systems, the PPG can also be used to monitor breathing, hypovolemia, and other circulatory conditions.[5] Additionally, the shape of the PPG waveform differs from subject to subject, and varies with the location and manner in which the pulse oximeter is attached.
Although PPG sensors are in common use in a number of commercial and clinical applications, the exact mechanisms determining the shape of the PPG waveform are not yet fully understood.[6]
Sites for measuring PPG
While pulse oximeters are commonly used medical devices, the PPG signal they record is rarely displayed and is nominally only processed to determine blood oxygenation and heart rate.[2] The PPG can be obtained from transmissive absorption (as at the finger tip) or reflection (as on the forehead).[2]
In outpatient settings, pulse oximeters are commonly worn on the finger. However, in cases of shock, hypothermia, etc., blood flow to the periphery can be reduced, resulting in a PPG without a discernible cardiac pulse.[7] In this case, a PPG can be obtained from a pulse oximeter on the head, with the most common sites being the ear, nasal septum, and forehead. PPG can also be configured for multi-site photoplethysmography (MPPG), e.g. by making simultaneous measurements from the right and left ear lobes, index fingers and great toes, and offering further opportunities for the assessment of patients with suspected peripheral arterial disease, autonomic dysfunction, endothelial dysfunction, and arterial stiffness. MPPG also offers significant potential for data mining, e.g. using deep learning, as well as a range of other innovative pulse wave analysis techniques.[8][9][10][11]
Motion artifacts are often a limiting factor preventing accurate readings during exercise and free living conditions.[6]
Uses
Monitoring heart rate and cardiac cycle
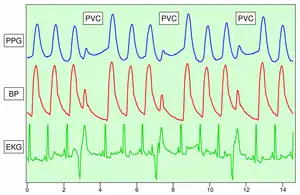
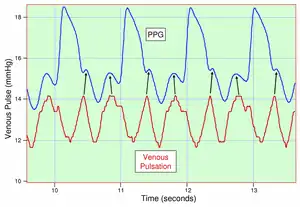
Because the skin is so richly perfused, it is relatively easy to detect the pulsatile component of the cardiac cycle. The DC component of the signal is attributable to the bulk absorption of the skin tissue, while the AC component is directly attributable to variation in blood volume in the skin caused by the pressure pulse of the cardiac cycle.
The height of AC component of the photoplethysmogram is proportional to the pulse pressure, the difference between the systolic and diastolic pressure in the arteries. As seen in the figure showing premature ventricular contractions (PVCs), the PPG pulse for the cardiac cycle with the PVC results in lower amplitude blood pressure and a PPG. Ventricular tachycardia and ventricular fibrillation can also be detected.[12]
Monitoring respiration
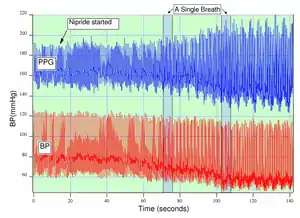
Respiration affects the cardiac cycle by varying the intrapleural pressure, the pressure between the thoracic wall and the lungs. Since the heart resides in the thoracic cavity between the lungs, the partial pressure of inhaling and exhaling greatly influence the pressure on the vena cava and the filling of the right atrium.
During inspiration, intrapleural pressure decreases by up to 4 mm Hg, which distends the right atrium, allowing for faster filling from the vena cava, increasing ventricular preload, but decreasing stroke volume. Conversely during expiration, the heart is compressed, decreasing cardiac efficiency and increasing stroke volume. When the frequency and depth of respiration increases, the venous return increases, leading to increased cardiac output.[14]
Much research has focused on estimating respiratory rate from the photoplethysmogram,[15] as well as more detailed respiratory measurements such as inspiratory time.[16]
Monitoring depth of anesthesia
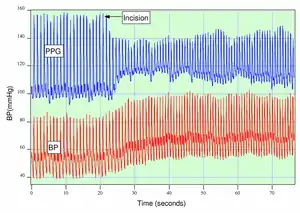
Anesthesiologists must often judge subjectively whether a patient is sufficiently anesthetized for surgery. As seen in the figure, if a patient is not sufficiently anesthetized, the sympathetic nervous system response to an incision can generate an immediate response in the amplitude of the PPG.[13]
Monitoring hypo- and hypervolemia
Shamir, Eidelman, et al. studied the interaction between inspiration and removal of 10% of a patient’s blood volume for blood banking before surgery.[17] They found that blood loss could be detected both from the photoplethysmogram from a pulse oximeter and an arterial catheter. Patients showed a decrease in the cardiac pulse amplitude caused by reduced cardiac preload during exhalation when the heart is being compressed.
Remote photoplethysmography
Conventional imaging
While photoplethysmography commonly requires some form of contact with the human skin (e.g., ear, finger), remote photoplethysmography allows physiological processes such as blood flow to be determined without skin contact. This is achieved by using face video to analyze subtle momentary changes in the subject's skin color which are not detectable to the human eye.[19][20] Such camera-based measurement of blood oxygen levels provides a contactless alternative to conventional photoplethysmography. For instance, it can be used to monitor the heart rate of newborn babies,[21] or analyzed with deep neural networks to quantify stress levels.[11]
Digital holography
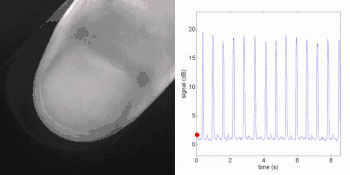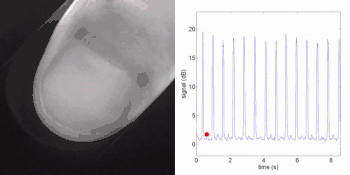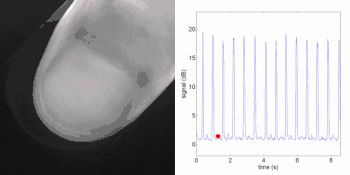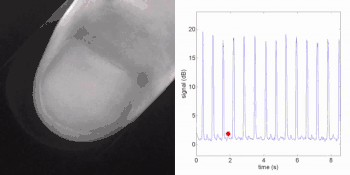
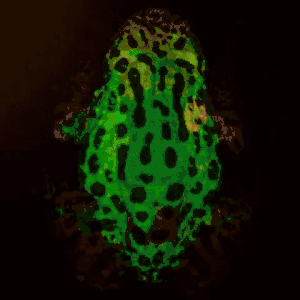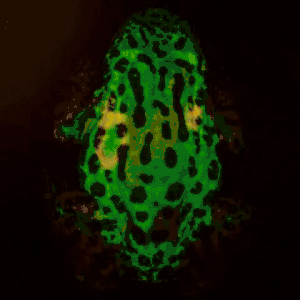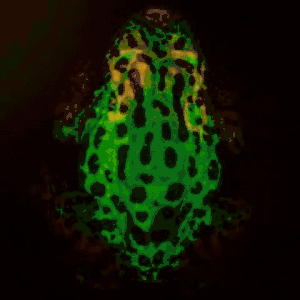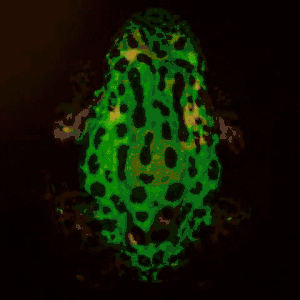
Remote photoplethysmography can also be performed by digital holography, which is sensitive to the phase of light waves, and hence can reveal sub-micron out-of-plane motion. In particular, wide-field imaging of pulsatile motion induced by blood flow can be measured on the thumb by digital holography. The results are comparable to blood pulse monitored by plethysmography during an occlusion-reperfusion experiment.[22] A major advantage of this system is that no physical contact with the studied tissue surface area is required. The two major limitations of this approach are (i) the off-axis interferometric configuration that reduces the available spatial bandwidth of the sensor array, and (ii) the use of short-time Fourier transform (via discrete Fourier transform) analysis that filters-off physiological signals.
Principal component analysis of digital holograms [23] reconstructed from digitized interferograms acquired at rates beyond ~1000 frames per second reveals surface waves on the hand. This method is an efficient way of performing digital holography from on-axis interferograms, which alleviates both the spatial bandwidth reduction of the off-axis configuration and the filtering of physiological signals. A higher spatial bandwidth is crucial for larger image field of view.
A refinement of holographic photoplethysmography, holographic laser Doppler imaging, enables non-invasive blood flow pulse wave monitoring in blood vessels of the retina, choroid, conjunctiva, and iris.[24] In particular, laser Doppler holography of the eye fundus, the choroid constitutes the predominant contribution to the high frequency laser Doppler signal. It is however possible to circumvent its influence by subtracting the spatially averaged baseline signal, and achieve high temporal resolution and full-field imaging capability of pulsatile blood flow.
See also
References
- Allen J (March 2007). "Photoplethysmography and its application in clinical physiological measurement". Physiological Measurement. 28 (3): R1–39. doi:10.1088/0967-3334/28/3/R01. PMID 17322588. S2CID 11450080.
- Kyriacou PA, Allen J, eds. (2021). Photoplethysmography: Technology, Signal Analysis and Applications. Elsevier.
- Shelley K, Shelley S, Lake C (2001). "Pulse Oximeter Waveform: Photoelectric Plethysmography". In Lake C, Hines R, Blitt C (eds.). Clinical Monitoring. W.B. Saunders Company. pp. 420–428.
- Pelaez EA, Villegas ER (2007). "LED power reduction trade-offs for ambulatory pulse oximetry". 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. Vol. 2007. pp. 2296–2299. doi:10.1109/IEMBS.2007.4352784. ISBN 978-1-4244-0787-3. PMID 18002450. S2CID 34626885.
- Reisner A, Shaltis PA, McCombie D, Asada HH (May 2008). "Utility of the photoplethysmogram in circulatory monitoring". Anesthesiology. 108 (5): 950–958. doi:10.1097/ALN.0b013e31816c89e1. PMID 18431132.
- Charlton PH, Kyriaco PA, Mant J, Marozas V, Chowienczyk P, Alastruey J (March 2022). "Wearable Photoplethysmography for Cardiovascular Monitoring". Proceedings of the IEEE. Institute of Electrical and Electronics Engineers. 110 (3): 355–381. doi:10.1109/JPROC.2022.3149785. PMC 7612541. PMID 35356509.
- Budidha K, Kyriacou PA (2015). "Investigation of photoplethysmography and arterial blood oxygen saturation from the ear-canal and the finger under conditions of artificially induced hypothermia" (PDF). 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). Vol. 2015. pp. 7954–7957. doi:10.1109/EMBC.2015.7320237. ISBN 978-1-4244-9271-8. PMID 26738137. S2CID 4574235.
- Allen J, Overbeck K, Nath AF, Murray A, Stansby G (April 2008). "A prospective comparison of bilateral photoplethysmography versus the ankle-brachial pressure index for detecting and quantifying lower limb peripheral arterial disease". Journal of Vascular Surgery. 47 (4): 794–802. doi:10.1016/j.jvs.2007.11.057. PMID 18381141.
- McKay ND, Griffiths B, Di Maria C, Hedley S, Murray A, Allen J (October 2014). "Novel photoplethysmography cardiovascular assessments in patients with Raynaud's phenomenon and systemic sclerosis: a pilot study". Rheumatology. 53 (10): 1855–1863. doi:10.1093/rheumatology/keu196. PMID 24850874.
- Mizeva I, Di Maria C, Frick P, Podtaev S, Allen J (March 2015). "Quantifying the correlation between photoplethysmography and laser Doppler flowmetry microvascular low-frequency oscillations". Journal of Biomedical Optics. 20 (3): 037007. Bibcode:2015JBO....20c7007M. doi:10.1117/1.JBO.20.3.037007. PMID 25764202. S2CID 206437523.
- Al-Jebrni AH, Chwyl B, Wang XY, Wong A, Saab BJ (2020-05-01). "AI-enabled remote and objective quantification of stress at scale". Biomedical Signal Processing and Control. 59: 101929. doi:10.1016/j.bspc.2020.101929. ISSN 1746-8094.
- Alian AA, Shelley KH (December 2014). "Photoplethysmography". Best Practice & Research. Clinical Anaesthesiology. 28 (4): 395–406. doi:10.1016/j.bpa.2014.08.006. PMID 25480769.
- Shelley KH (December 2007). "Photoplethysmography: beyond the calculation of arterial oxygen saturation and heart rate". Anesthesia and Analgesia. 105 (6 Suppl): S31–S36. doi:10.1213/01.ane.0000269512.82836.c9. PMID 18048895. S2CID 21556782.
- Shelley KH, Jablonka DH, Awad AA, Stout RG, Rezkanna H, Silverman DG (August 2006). "What is the best site for measuring the effect of ventilation on the pulse oximeter waveform?". Anesthesia and Analgesia. 103 (2): 372–7, table of contents. doi:10.1213/01.ane.0000222477.67637.17. PMID 16861419. S2CID 6926327.
- Charlton PH, Birrenkott DA, Bonnici T, Pimentel MA, Johnson AE, Alastruey J, et al. (2018). "Breathing Rate Estimation From the Electrocardiogram and Photoplethysmogram: A Review". IEEE Reviews in Biomedical Engineering. 11: 2–20. doi:10.1109/RBME.2017.2763681. PMC 7612521. PMID 29990026.
- Davies HJ, Bachtiger P, Williams I, Molyneaux PL, Peters NS, Mandic DP (July 2022). "Wearable In-Ear PPG: Detailed Respiratory Variations Enable Classification of COPD". IEEE Transactions on Bio-Medical Engineering. 69 (7): 2390–2400. doi:10.1109/TBME.2022.3145688. hdl:10044/1/96220. PMID 35077352. S2CID 246287257.
- Shamir M, Eidelman LA, Floman Y, Kaplan L, Pizov R (February 1999). "Pulse oximetry plethysmographic waveform during changes in blood volume". British Journal of Anaesthesia. 82 (2): 178–81. doi:10.1093/bja/82.2.178. PMID 10364990.
- Wendling P (28 August 2019). "FDA Okays Biobeat's Cuffless Blood Pressure Monitor". Medscape. Retrieved 5 September 2019.
- Verkruysse W, Svaasand LO, Nelson JS (December 2008). "Remote plethysmographic imaging using ambient light". Optics Express. 16 (26): 21434–21445. Bibcode:2008OExpr..1621434V. doi:10.1364/OE.16.021434. PMC 2717852. PMID 19104573.
- Rouast PV, Adam MT, Chiong R, Cornforth D, Lux E (2018). "Remote heart rate measurement using low-cost RGB face video: A technical literature review". Frontiers of Computer Science. 12 (5): 858–872. doi:10.1007/s11704-016-6243-6. S2CID 1483621.
- Example of contactless monitoring in action. YouTube. Archived from the original on 2021-12-11.
- Bencteux J, Pagnoux P, Kostas T, Bayat S, Atlan M (June 2015). "Holographic laser Doppler imaging of pulsatile blood flow". Journal of Biomedical Optics. 20 (6): 066006. arXiv:1501.05776. Bibcode:2015JBO....20f6006B. doi:10.1117/1.JBO.20.6.066006. PMID 26085180. S2CID 20234484.
- Puyo L, Bellonnet-Mottet L, Martin A, Te F, Paques M, Atlan M (2020). "Real-time digital holography of the retina by principal component analysis". arXiv:2004.00923 [physics.med-ph].
- Puyo L, Paques M, Fink M, Sahel JA, Atlan M (September 2018). "In vivo laser Doppler holography of the human retina". Biomedical Optics Express. 9 (9): 4113–4129. arXiv:1804.10066. doi:10.1364/BOE.9.004113. PMC 6157768. PMID 30615709.