Phylogenetics
In biology, phylogenetics (/ˌfaɪloʊdʒəˈnɛtɪks, -lə-/)[1][2][3] is the study of the evolutionary history and relationships among or within groups of organisms. These relationships are determined by phylogenetic inference methods that focus on observed heritable traits, such as DNA sequences, protein amino acid sequences, or morphology. The result of such an analysis is a phylogenetic tree—a diagram containing a hypothesis of relationships that reflects the evolutionary history of a group of organisms.[4]
| Part of a series on |
| Evolutionary biology |
|---|
 |
|
The tips of a phylogenetic tree can be living taxa or fossils, and represent the "end" or the present time in an evolutionary lineage. A phylogenetic diagram can be rooted or unrooted. A rooted tree diagram indicates the hypothetical common ancestor of the tree. An unrooted tree diagram (a network) makes no assumption about the ancestral line, and does not show the origin or "root" of the taxa in question or the direction of inferred evolutionary transformations.[5]
In addition to their use for inferring phylogenetic patterns among taxa, phylogenetic analyses are often employed to represent relationships among genes or individual organisms. Such uses have become central to understanding biodiversity, evolution, ecology, and genomes.
Phylogenetics is component of systematics that uses similarities and differences of the characteristics of species to interpret their evolutionary relationships and origins. Phylogenetics focuses on whether the characteristics of a species reinforce a phylogenetic inference that it diverged from the most recent common ancestor of a taxonomic group.[6]
In the field of cancer research, phylogenetics can be used to study the clonal evolution of tumors and molecular chronology, predicting and showing how cell populations vary throughout the progression of the disease and during treatment, using whole genome sequencing techniques.[7] The evolutionary processes behind cancer progression are quite different from those in species and are important to phylogenetic inference; these differences manifest in at least four areas: the types of aberrations that occur, the rates of mutation, the intensity, and high heterogeneity - variability - of tumor cell subclones.[8]
Phylogenetics can also aid in drug design and discovery. Phylogenetics allows scientists to organize species and can show which species are likely to have inherited particular traits that are medically useful, such as producing biologically active compounds - those that have effects on the human body. For example, in drug discovery, venom-producing animals are particularly useful. Venoms from these animals produce several important drugs, e.g., ACE inhibitors and Prialt (Ziconotide). To find new venoms, scientists turn to phylogenetics to screen for closely related species that may have the same useful traits. The phylogenetic tree shows which species of fish have an origin of venom, and related fish they may contain the trait. Using this approach in studying venomous fish, biologists are able to identify the fish species that may be venomous. Biologist have used this approach in many species such as snakes and lizards.[9] In forensic science, phylogenetic tools are useful to assess DNA evidence for court cases. The simple phylogenetic tree of viruses A-E shows the relationships between viruses e.g., all viruses are descendants of Virus A.
HIV forensics uses phylogenetic analysis to track the differences in HIV genes and determine the relatedness of two samples. Phylogenetic analysis has been used in criminal trials to exonerate or hold individuals. HIV forensics does have its limitations, i.e., it cannot be the sole proof of transmission between individuals and phylogenetic analysis which shows transmission relatedness does not indicate direction of transmission.[10]
Taxonomy and classification

Taxonomy is the identification, naming, and classification of organisms. Compared to systemization, classification emphasizes whether a species has characteristics of a taxonomic group.[6] The Linnaean classification system developed in the 1700s by Carolus Linnaeus is the foundation for modern classification methods. Linnaean classification relies on an organism's phenotype or physical characteristics to group and organize species.[11] With the emergence of biochemistry, organism classifications are now usually based on phylogenetic data, and many systematists contend that only monophyletic taxa should be recognized as named groups. The degree to which classification depends on inferred evolutionary history differs depending on the school of taxonomy: phenetics ignores phylogenetic speculation altogether, trying to represent the similarity between organisms instead; cladistics (phylogenetic systematics) tries to reflect phylogeny in its classifications by only recognizing groups based on shared, derived characters (synapomorphies); evolutionary taxonomy tries to take into account both the branching pattern and "degree of difference" to find a compromise between them.
Inference of a phylogenetic tree
Usual methods of phylogenetic inference involve computational approaches implementing the optimality criteria and methods of parsimony, maximum likelihood (ML), and MCMC-based Bayesian inference. All these depend upon an implicit or explicit mathematical model describing the evolution of characters observed.
Phenetics, popular in the mid-20th century but now largely obsolete, used distance matrix-based methods to construct trees based on overall similarity in morphology or similar observable traits (i.e. in the phenotype or the overall similarity of DNA, not the DNA sequence), which was often assumed to approximate phylogenetic relationships.
Prior to 1950, phylogenetic inferences were generally presented as narrative scenarios. Such methods are often ambiguous and lack explicit criteria for evaluating alternative hypotheses.[12][13][14]
Impacts of taxon sampling
In phylogenetic analysis, taxon sampling selects a small group of taxa to represent the evolutionary history of its broader population.[15] This process is also known as stratified sampling or clade-based sampling.[16] The practice occurs given limited resources to compare and analyze every species within a target population.[15] Based on the representative group selected, the construction and accuracy of phylogenetic trees vary, which impacts derived phylogenetic inferences.[16]
Unavailable datasets, such as an organism's incomplete DNA and protein amino acid sequences in genomic databases, directly restrict taxonomic sampling.[16] Consequently, a significant source of error within phylogenetic analysis occurs due to inadequate taxon samples. Accuracy may be improved by increasing the number of genetic samples within its monophyletic group. Conversely, increasing sampling from outgroups extraneous to the target stratified population may decrease accuracy. Long branch attraction is an attributed theory for this occurrence, where nonrelated branches are incorrectly classified together, insinuating a shared evolutionary history.[15]

There are debates if increasing the number of taxa sampled improves phylogenetic accuracy more than increasing the number of genes sampled per taxon. Differences in each method's sampling impact the number of nucleotide sites utilized in a sequence alignment, which may contribute to disagreements. For example, phylogenetic trees constructed utilizing a more significant number of total nucleotides are generally more accurate, as supported by phylogenetic trees' bootstrapping replicability from random sampling.
The graphic presented in Taxon Sampling, Bioinformatics, and Phylogenomics, compares the correctness of phylogenetic trees generated using fewer taxa and more sites per taxon on the x-axis to more taxa and fewer sites per taxon on the y-axis. With fewer taxa, more genes are sampled amongst the taxonomic group; in comparison, with more taxa added to the taxonomic sampling group, fewer genes are sampled. Each method has the same total number of nucleotide sites sampled. Furthermore, the dotted line represents a 1:1 accuracy between the two sampling methods. As seen in the graphic, most of the plotted points are located below the dotted line, which indicates gravitation toward increased accuracy when sampling fewer taxa with more sites per taxon. The research performed utilizes four different phylogenetic tree construction models to verify the theory; neighbor-joining (NJ), minimum evolution (ME), unweighted maximum parsimony (MP), and maximum likelihood (ML). In the majority of models, sampling fewer taxon with more sites per taxon demonstrated higher accuracy.
Generally, with the alignment of a relatively equal number of total nucleotide sites, sampling more genes per taxon has higher bootstrapping replicability than sampling more taxa. However, unbalanced datasets within genomic databases make increasing the gene comparison per taxon in uncommonly sampled organisms increasingly difficult.[16]
History
Overview
The term "phylogeny" derives from the German Phylogenie, introduced by Haeckel in 1866,[17] and the Darwinian approach to classification became known as the "phyletic" approach.[18] It can be traced back to Aristotle, who wrote in his Posterior Analytics, "We may assume the superiority ceteris paribus [other things being equal] of the demonstration which derives from fewer postulates or hypotheses."
Ernst Haeckel's recapitulation theory
The modern concept of phylogenetics evolved primarily as a disproof of a previously widely accepted theory. During the late 19th century, Ernst Haeckel's recapitulation theory, or "biogenetic fundamental law", was widely accepted. It was often expressed as "ontogeny recapitulates phylogeny", i.e. the development of a single organism during its lifetime, from germ to adult, successively mirrors the adult stages of successive ancestors of the species to which it belongs. But this theory has long been rejected.[19][20] Instead, ontogeny evolves – the phylogenetic history of a species cannot be read directly from its ontogeny, as Haeckel thought would be possible, but characters from ontogeny can be (and have been) used as data for phylogenetic analyses; the more closely related two species are, the more apomorphies their embryos share.
Timeline of key points


- 14th century, lex parsimoniae (parsimony principle), William of Ockam, English philosopher, theologian, and Franciscan friar, but the idea actually goes back to Aristotle, as a precursor concept. He introduced the concept of Occam's razor, which is the problem solving principle that recommends searching for explanations constructed with the smallest possible set of elements. Though he did not use these exact words, the principle can be summarized as "Entities must not be multiplied beyond necessity." The principle advocates that when presented with competing hypotheses about the same prediction, one should prefer the one that requires fewest assumptions.
- 1763, Bayesian probability, Rev. Thomas Bayes,[21] a precursor concept. Bayesian probability began a resurgence in the 1950's, allowing scientists in the computing field to pair traditional Bayesian statistics with other more modern techniques. It is now used as a blanket term for several related interpretations of probability as an amount of epistemic confidence.
- 18th century, Pierre Simon (Marquis de Laplace), perhaps first to use ML (maximum likelihood), precursor concept. His work gave way to the Laplace distribution, which can be directly linked to least absolute deviations.
- 1809, evolutionary theory, Philosophie Zoologique, Jean-Baptiste de Lamarck, precursor concept, foreshadowed in the 17th century and 18th century by Voltaire, Descartes, and Leibniz, with Leibniz even proposing evolutionary changes to account for observed gaps suggesting that many species had become extinct, others transformed, and different species that share common traits may have at one time been a single race,[22] also foreshadowed by some early Greek philosophers such as Anaximander in the 6th century BC and the atomists of the 5th century BC, who proposed rudimentary theories of evolution[23]
- 1837, Darwin's notebooks show an evolutionary tree[24]
- 1840, American Geologist Edward Hitchcock published what is considered to be the first paleontological "Tree of Life". Many critiques, modifications, and explanations would follow.[25]
 This chart displays one of the first published attempts at a paleontological "Tree of Life" by Geologist Edward Hitchcock. (1840)
This chart displays one of the first published attempts at a paleontological "Tree of Life" by Geologist Edward Hitchcock. (1840) - 1843, distinction between homology and analogy (the latter now referred to as homoplasy), Richard Owen, precursor concept. Homology is the term used to characterize the similarity of features that can be parsimoniously explained by common ancestry. Homoplasy is the term used to describe a feature that has been gained or lost independently in separate lineages over the course of evolution.
- 1858, Paleontologist Heinrich Georg Bronn (1800–1862) published a hypothetical tree to illustrating the paleontological "arrival" of new, similar species following the extinction of an older species. Bronn did not propose a mechanism responsible for such phenomena, precursor concept.[26]
- 1858, elaboration of evolutionary theory, Darwin and Wallace,[27] also in Origin of Species by Darwin the following year, precursor concept
- 1866, Ernst Haeckel, first publishes his phylogeny-based evolutionary tree, precursor concept. Haeckel introduces the now-disproved recapitulation theory.
- 1893, Dollo's Law of Character State Irreversibility,[28] precursor concept. Dollo's Law of Irreversibility states that "an organism never comes back exactly to its previous state due to the indestructible nature of the past, it always retains some trace of the transitional stages through which it has passed."[29]
- 1912, ML (maximum likelihood recommended, analyzed, and popularized by Ronald Fisher, precursor concept. Fisher is one of the main contributors to the early 20th-century revival of Darwinism, and has been called the "greatest of Darwin's successors" for his contributions to the revision of the theory of evolution and his use of mathematics to combine Mendelian genetics and natural selection in the 20th century "modern synthesis".
- 1921, Tillyard uses term "phylogenetic" and distinguishes between archaic and specialized characters in his classification system[30]
- 1940, term "clade" coined by Lucien Cuénot
- 1949, Jackknife resampling, Maurice Quenouille (foreshadowed in '46 by Mahalanobis and extended in '58 by Tukey), precursor concept
- 1950, Willi Hennig's classic formalization.[31] Hennig is considered the founder of phylogenetic systematics, and published his first works in German of this year. He also asserted a version of the parsimony principle, stating that the presence of amorphous characters in different species 'is always reason for suspecting kinship, and that their origin by convergence should not be presumed a priori'. This has been considered a foundational view of phylogenetic inference.
- 1952, William Wagner's ground plan divergence method[32]
- 1953, "cladogenesis" coined[33]
- 1960, "cladistic" coined by Cain and Harrison[34]
- 1963, first attempt to use ML (maximum likelihood) for phylogenetics, Edwards and Cavalli-Sforza[35]
- 1965
- Camin-Sokal parsimony, first parsimony (optimization) criterion and first computer program/algorithm for cladistic analysis both by Camin and Sokal[36]
- character compatibility method, also called clique analysis, introduced independently by Camin and Sokal (loc. cit.) and E. O. Wilson[37]
- 1966
- English translation of Hennig[38]
- "cladistics" and "cladogram" coined (Webster's, loc. cit.)
- 1969
- 1970, Wagner parsimony generalized by Farris[42]
- 1971
- first successful application of ML (maximum likelihood) to phylogenetics (for protein sequences), Neyman[43]
- Fitch parsimony, Walter M. Fitch.[44] These gave way to the most basic ideas of maximum parsimony. Fitch is known for his work on reconstructing phylogenetic trees from protein and DNA sequences. His definition of orthologous sequences has been referenced in many research publications.
- NNI (nearest neighbour interchange), first branch-swapping search strategy, developed independently by Robinson[45] and Moore et al.
- ME (minimum evolution), Kidd and Sgaramella-Zonta[46] (it is unclear if this is the pairwise distance method or related to ML as Edwards and Cavalli-Sforza call ML "minimum evolution")
- 1972, Adams consensus, Adams[47]
- 1976, prefix system for ranks, Farris[48]
- 1977, Dollo parsimony, Farris[49]
- 1979
- 1980, PHYLIP, first software package for phylogenetic analysis, Joseph Felsenstein. A free computational phylogenetics package of programs for inferring evolutionary trees (phylogenies). One such example tree created by PHILYP, called a "drawgram", generates rooted trees. This image shown in the figure below shows the evolution of phylogenetic trees over time.
- 1981
- majority consensus, Margush and MacMorris[53]
- strict consensus, Sokal and Rohlf[54]first computationally efficient ML (maximum likelihood) algorithm.[55] Felsenstein created the Felsenstein Maximum Likelihood method, used for the inference of phylogeny which evaluates a hypothesis about evolutionary history in terms of the probability that the proposed model and the hypothesized history would give rise to the observed data set.
 This image depicts a PHILYP generated drawgram. This drawgram is an example of one of the possible trees the software is capable of generating.
This image depicts a PHILYP generated drawgram. This drawgram is an example of one of the possible trees the software is capable of generating.
- 1982
- PHYSIS, Mikevich and Farris
- branch and bound, Hendy and Penny[56]
- 1985
- 1986, MacClade, Maddison and Maddison
- 1987, neighbor-joining method Saitou and Nei[60]
- 1988, Hennig86 (version 1.5), Farris
- Bremer support (decay index), Bremer[61]
- 1989
- 1990
- 1991
- 1993, implied weighting Goloboff[68]
- 1994, reduced consensus: RCC (reduced cladistic consensus) for rooted trees, Wilkinson[69]
- 1995, reduced consensus RPC (reduced partition consensus) for unrooted trees, Wilkinson[70]
- 1996, first working methods for BI (Bayesian Inference) independently developed by Li,[71] Mau,[72] and Rannala and Yang[73] and all using MCMC (Markov chain-Monte Carlo)
- 1998, TNT (Tree Analysis Using New Technology), Goloboff, Farris, and Nixon
- 1999, Winclada, Nixon
- 2003, symmetrical resampling, Goloboff[74]
- 2004, 2005, similarity metric (using an approximation to Kolmogorov complexity) or NCD (normalized compression distance), Li et al.,[75] Cilibrasi and Vitanyi.[76]
Outside biology

Phylogenetic tools and representations (trees and networks) can also be applied philology, the study of the evolution of oral languages and written text and manuscripts, such as in the field of quantitative comparative linguistics.[78]
Computational phylogenetics can be used to investigate a language as an evolutionary system. The evolution of human language closely corresponds with human's biological evolution which allows phylogenetic methods to be applied. The concept of a "tree" serves as an efficient way to represent relationships between languages and language splits. It also serves as a way of testing hypotheses about the connections and ages of language families. For example, relationships among languages can be shown by using cognates as characters.[79][80] The phylogenetic tree of Indo-European languages shows the relationships between several of the languages in a timeline, as well as the similarity between words and word order.
There are three types of criticisms about using phylogenetics in philology, the first arguing that languages and species are different entities, therefore you can not use the same methods to study both. The second being how phylogenetic methods are being applied to linguistic data. And the third, discusses the types of data that is being used to construct the trees.[79]
Bayesian phylogenetic methods, which are sensitive to how treelike the data is, allow for the reconstruction of relationships among languages, locally and globally. The main two reasons for the use of Bayesian phylogenetics are that (1) diverse scenarios can be included in calculations and (2) the output is a sample of trees and not a single tree with true claim.[81]
The same process can be applied to texts and manuscripts. In Paleography, the study of historical writings and manuscripts, texts were replicated by scribes who copied from their source and alterations - i.e., 'mutations' - occurred when the scribe did not precisely copy the source.[82]
Phylogenetic Screening in the role of Biodiversity
Phylogenetic screens involve the pharmacological examination of closely related groups of organisms. Advances in cladistic analysis through rapid computer programs and molecular techniques have improved the precision of phylogenetic determination, allowing for the identification of species with pharmacological potential. Phylogenetic screens have been used in a rudimentary manner in the past, such as studying the Apocynaceae family of plants known for their alkaloid-producing species like Catharanthus, which produces vincristine, an antileukemia drug. However, modern techniques now enable researchers to study close relatives of a species to uncover either (1) higher abundance of important bioactive compounds (e.g., species of Taxus for taxol) or (2) natural variants of known pharmaceuticals (e.g., species of Catharanthus for different forms of vincristine or vinblastine.
Looking at Fig 6. it contains the phylogenetic screen of biodiversity within the fungi family. As seen inside the circle there are subtrees present that were done via phylogenetic analysis. These relations help understand the evolutionary history of various groups of organisms, identifying relationships between different species, and predicting future evolutionary changes. If we were to take biodiversity information from existing knowledge there might be relations between species or subgroups that we didnt know. But with emerging imagery systems and new analysis techniques more genetic relation can be found in biodiverse fields. The image below can help with conservation efforts as there are rare species of fungi involved, that could be beneficial to ecosystems all around.[83]

Phylogenetic tree shapes Insight on Disease Transmission Patterns
Whole-genome sequence data of pathogens obtained from outbreaks or epidemics of infectious diseases can provide important insights into transmission dynamics and inform public health strategies. Previous studies have relied on integrating genomic and epidemiological data to reconstruct transmission events. However, recent research has explored the possibility of deducing transmission patterns solely from genomic data using phylodynamics, which involves analyzing the properties of pathogen phylogenies. Phylodynamics uses theoretical models to compare predicted branch lengths with actual branch lengths in phylogenies to infer transmission patterns. Additionally, coalescent theory, which describes probability distributions on trees based on population size, has been adapted for epidemiological purposes. Another potential source of information within phylogenies that has been explored is "tree shape". These approaches are computationally intensive but have the potential to provide valuable insights into pathogen transmission dynamics.

The structure of the host contact network has a profound impact on the dynamics of outbreaks or epidemics, and outbreak management strategies rely on the type of transmission patterns driving the outbreak. One can expect that pathogen genomes spreading through different contact network structures, such as chains, homogenous networks, or networks with super-spreaders, would accumulate mutations in distinct patterns, resulting in noticeable differences in the shape of phylogenetic trees, as illustrated in Fig. 1. Analyzation of the structural characteristics of phylogenetic trees generated from simulated bacterial genome evolution across multiple types of contact networks was conducted. Simple topological properties of phylogenetic trees that, when combined, can be used to classify trees into chain-like, homogenous, or super-spreading dynamics, revealing transmission dynamics. These properties form the basis of a computational classifier are used to classify real-world outbreaks. Remarkably, the computational predictions of overall transmission dynamics for each outbreak align with known epidemiology [84]
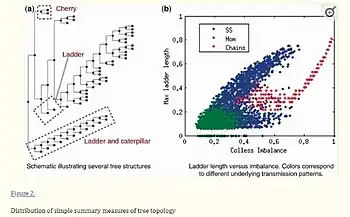
Different transmission networks result in quantitatively different tree shapes To determine whether tree shapes captured information about the underlying disease transmission patterns within an outbreak, we simulated evolution of a bacterial genome over three types of outbreak contact network—homogenous, super-spreading and chain—and summarized the resulting phylogenies with five metrics describing tree shape. Figure 2 and and33 illustrate the distributions of these metrics across the three types of outbreaks, revealing clear differences in tree topology depending on the underlying host contact network. Super-spreader networks gave rise to phylogenies with higher Colless imbalance, longer ladder patterns, lower Δw and deeper trees than transmission networks with a homogeneous distribution of contacts. Trees derived from chain-like networks were less variable, deeper, more imbalanced and narrower than the other trees. Other topological summary metrics considered did not resolve the three outbreak types as fully (Supplementary Information). Scatter plots can be used for pathogen transmission analysis to visualize the relationship between two variables, such as the number of infected individuals and the time since infection. For example, a scatter plot can be used to examine the relationship between the number of cases of a pathogen and the amount of time since the first case was reported. This can help to identify trends and patterns in the data, such as whether the spread of the pathogen is increasing or decreasing over time. Scatter plots can also be used to identify any outliers or clusters of data points, which can provide insight into potential transmission routes or super-spreader events. Overall, scatter plots can be a useful tool in pathogen transmission analysis to identify patterns and trends in the data, and to inform public health interventions and control measures.[84]

The box plot imagery on the right displays the pathogen transformation data. Box plots are often used in statistical analysis to compare different groups or to visualize changes in a single group over time. They are particularly useful when dealing with large datasets or when comparing several groups, as they can quickly highlight differences or similarities in the data. Box plots, also known as box-and-whisker plots, are useful in statistical analysis to provide a summary of the distribution of a dataset. They display the range, median, quartiles, and potential outliers of the data in a visual manner. Box plots are commonly used to compare different groups or to analyze changes in a single group over time. They are especially helpful when working with large datasets or when comparing multiple groups, as they can easily identify any differences or similarities in the data. This makes box plots a valuable tool for analyzing pathogen transmission data, as they can help to identify important features in the distribution of the data.[84]
See also
- Angiosperm Phylogeny Group
- Bauplan
- Bioinformatics
- Biomathematics
- Coalescent theory
- EDGE of Existence programme
- Evolutionary taxonomy
- Language family
- Maximum parsimony
- Microbial phylogenetics
- Molecular phylogeny
- Noogenesis
- Ontogeny
- PhyloCode
- Phylodynamics
- Phylogenesis
- Phylogenetic comparative methods
- Phylogenetic network
- Phylogenetic nomenclature
- Phylogenetic tree viewers
- Phylogenetics software
- Phylogenomics
- Phylogeny (psychoanalysis)
- Phylogeography
- Systematics
References
- "phylogenetic". Dictionary.com Unabridged (Online). n.d.
- "phylogenetic". Merriam-Webster.com Dictionary.
- from Greek φυλή/φῦλον [phylé/phylon] "tribe, clan, race", and γενετικός [genetikós] "origin, source, birth")Liddell, Henry George; Scott, Robert; Jones, Henry Stuart (1968). A Greek-English lexicon (9 ed.). Oxford: Clarendon Press. p. 1961.
- "phylogeny". Biology online. Retrieved 15 February 2013.
- Itzik, Peer (1 January 2001). "Phylogenetic Trees". www.cs.tau.ac.il.
- Harris, Katherine (23 June 2019). Taxonomy & Phylogeny. Biology LibreTexts. Retrieved 19 April 2023.
- Herberts, Cameron; Annala, Matti; Sipola, Joonatan; Ng, Sarah W. S.; Chen, Xinyi E.; Nurminen, Anssi; Korhonen, Olga V.; Munzur, Aslı D.; Beja, Kevin; Schönlau, Elena; Bernales, Cecily Q.; Ritch, Elie; Bacon, Jack V. W.; Lack, Nathan A.; Nykter, Matti (August 2022). "Deep whole-genome ctDNA chronology of treatment-resistant prostate cancer". Nature. 608 (7921): 199–208. Bibcode:2022Natur.608..199H. doi:10.1038/s41586-022-04975-9. ISSN 1476-4687. PMID 35859180. S2CID 250730778.
- Schwartz, Russell; Schäffer, Alejandro A. (April 2017). "The evolution of tumour phylogenetics: principles and practice". Nature Reviews Genetics. 18 (4): 213–229. doi:10.1038/nrg.2016.170. ISSN 1471-0056. PMC 5886015. PMID 28190876.
- "Drug discovery - Understanding Evolution". 7 July 2021. Retrieved 23 April 2023.
- Bernard, EJ; Azad, Y; Vandamme, AM; Weait, M; Geretti, AM (2007). "HIV forensics: pitfalls and acceptable standards in the use of phylogenetic analysis as evidence in criminal investigations of HIV transmission". HIV Medicine. 8 (6): 382–387. doi:10.1111/j.1468-1293.2007.00486.x. ISSN 1464-2662. PMID 17661846. S2CID 38883310.
- CK-12 Foundation (6 March 2021). Linnaean Classification. Biology LibreTexts. Retrieved 19 April 2023.
- Richard C. Brusca & Gary J. Brusca (2003). Invertebrates (2nd ed.). Sunderland, Massachusetts: Sinauer Associates. ISBN 978-0-87893-097-5.
- Bock, W. J. (2004). Explanations in systematics. Pp. 49–56. In Williams, D. M. and Forey, P. L. (eds) Milestones in Systematics. London: Systematics Association Special Volume Series 67. CRC Press, Boca Raton, Florida.
- Auyang, Sunny Y. (1998). Narratives and Theories in Natural History. In: Foundations of complex-system theories: in economics, evolutionary biology, and statistical physics. Cambridge, U.K.; New York: Cambridge University Press.
- Rosenberg, Michael (28 August 2001). "Incomplete taxon sampling is not a problem for phylogenetic inference". Proceedings of the National Academy of Sciences. 98 (19): 10751–10756. Bibcode:2001PNAS...9810751R. doi:10.1073/pnas.191248498. PMC 58547. PMID 11526218.
- Rosenberg, Michael; Kumar, Sudhir (1 February 2023). "Taxon Sampling, Bioinformatics, and Phylogenetics". Evolutionary Journal of the Linnean Society. 52 (1): 119–124. doi:10.1080/10635150390132894. PMC 2796430. PMID 12554445. Retrieved 19 April 2023.
- Harper, Douglas (2010). "Phylogeny". Online Etymology Dictionary.
- Stuessy 2009.
- Blechschmidt, Erich (1977) The Beginnings of Human Life. Springer-Verlag Inc., p. 32: "The so-called basic law of biogenetics is wrong. No buts or ifs can mitigate this fact. It is not even a tiny bit correct or correct in a different form, making it valid in a certain percentage. It is totally wrong."
- Ehrlich, Paul; Richard Holm; Dennis Parnell (1963) The Process of Evolution. New York: McGraw–Hill, p. 66: "Its shortcomings have been almost universally pointed out by modern authors, but the idea still has a prominent place in biological mythology. The resemblance of early vertebrate embryos is readily explained without resort to mysterious forces compelling each individual to reclimb its phylogenetic tree."
- Bayes, Mr; Price, Mr (1763). "An Essay towards Solving a Problem in the Doctrine of Chances. By the Late Rev. Mr. Bayes, F. R. S. Communicated by Mr. Price, in a Letter to John Canton, A. M. F. R. S". Philosophical Transactions of the Royal Society of London. 53: 370–418. doi:10.1098/rstl.1763.0053.
- Strickberger, Monroe. 1996. Evolution, 2nd. ed. Jones & Bartlett.
- The Theory of Evolution, Teaching Company course, Lecture 1
- Darwin's Tree of Life Archived 13 March 2014 at the Wayback Machine
- Archibald, J. David (1 August 2009). "Edward Hitchcock's Pre-Darwinian (1840) "Tree of Life"". Journal of the History of Biology. 42 (3): 561–592. doi:10.1007/s10739-008-9163-y. ISSN 1573-0387. PMID 20027787. S2CID 16634677.
- Archibald, J. David (2008). "Edward Hitchcock's Pre-Darwinian (1840) 'Tree of Life'". Journal of the History of Biology. 42 (3): 561–92. CiteSeerX 10.1.1.688.7842. doi:10.1007/s10739-008-9163-y. PMID 20027787. S2CID 16634677.
- Darwin, Charles; Wallace, Alfred (1858). "On the Tendency of Species to form Varieties; and on the Perpetuation of Varieties and Species by Natural Means of Selection". Journal of the Proceedings of the Linnean Society of London. Zoology. 3 (9): 45–62. doi:10.1111/j.1096-3642.1858.tb02500.x.
- Dollo, Louis. 1893. Les lois de l'évolution. Bull. Soc. Belge Géol. Paléont. Hydrol. 7: 164–66.
- Galis, Frietson; Arntzen, Jan W.; Lande, Russell (2010). "Dollo's Law and the Irreversibility of Digit Loss in Bachia". Evolution. 64 (8): 2466–76, discussion 2477-85. doi:10.1111/j.1558-5646.2010.01041.x. PMID 20500218. S2CID 24520027. Retrieved 23 April 2023.
- Tillyard, R. J (2012). "A New Classification of the Order Perlaria". The Canadian Entomologist. 53 (2): 35–43. doi:10.4039/Ent5335-2. S2CID 90171163.
- Hennig, Willi (1950). Grundzüge einer Theorie der Phylogenetischen Systematik [Basic features of a theory of phylogenetic systematics] (in German). Berlin: Deutscher Zentralverlag. OCLC 12126814.
- Wagner, Warren Herbert (1952). "The fern genus Diellia: structure, affinities, and taxonomy". University of California Publications in Botany. 26 (1–6): 1–212. OCLC 4228844.
- Webster's 9th New Collegiate Dictionary
- Cain, A. J; Harrison, G. A (2009). "Phyletic Weighting". Proceedings of the Zoological Society of London. 135 (1): 1–31. doi:10.1111/j.1469-7998.1960.tb05828.x.
- "The reconstruction of evolution" in "Abstracts of Papers". Annals of Human Genetics. 27 (1): 103–5. 1963. doi:10.1111/j.1469-1809.1963.tb00786.x.
- Camin, Joseph H; Sokal, Robert R (1965). "A Method for Deducing Branching Sequences in Phylogeny". Evolution. 19 (3): 311–26. doi:10.1111/j.1558-5646.1965.tb01722.x. S2CID 20957422.
- Wilson, Edward O (1965). "A Consistency Test for Phylogenies Based on Contemporaneous Species". Systematic Zoology. 14 (3): 214–20. doi:10.2307/2411550. JSTOR 2411550.
- Hennig. W. (1966). Phylogenetic systematics. Illinois University Press, Urbana.
- Farris, James S (1969). "A Successive Approximations Approach to Character Weighting". Systematic Zoology. 18 (4): 374–85. doi:10.2307/2412182. JSTOR 2412182.
- Kluge, A. G; Farris, J. S (1969). "Quantitative Phyletics and the Evolution of Anurans". Systematic Biology. 18 (1): 1–32. doi:10.1093/sysbio/18.1.1.
- Quesne, Walter J. Le (1969). "A Method of Selection of Characters in Numerical Taxonomy". Systematic Zoology. 18 (2): 201–205. doi:10.2307/2412604. JSTOR 2412604.
- Farris, J. S (1970). "Methods for Computing Wagner Trees". Systematic Biology. 19: 83–92. doi:10.1093/sysbio/19.1.83.
- Neyman, Jerzy (1971). "Molecular studies of evolution: a source of novel statistical problems". Statistical Decision Theory and Related Topics. pp. 1–27. doi:10.1016/B978-0-12-307550-5.50005-8. ISBN 978-0-12-307550-5.
- Fitch, W. M (1971). "Toward Defining the Course of Evolution: Minimum Change for a Specific Tree Topology". Systematic Biology. 20 (4): 406–16. doi:10.1093/sysbio/20.4.406. JSTOR 2412116.
- Robinson, D.F (1971). "Comparison of labeled trees with valency three". Journal of Combinatorial Theory. Series B. 11 (2): 105–19. doi:10.1016/0095-8956(71)90020-7.
- Kidd, K. K; Sgaramella-Zonta, L. A (1971). "Phylogenetic analysis: Concepts and methods". American Journal of Human Genetics. 23 (3): 235–52. PMC 1706731. PMID 5089842.
- Adams, E. N (1972). "Consensus Techniques and the Comparison of Taxonomic Trees". Systematic Biology. 21 (4): 390–397. doi:10.1093/sysbio/21.4.390.
- Farris, James S (1976). "Phylogenetic Classification of Fossils with Recent Species". Systematic Zoology. 25 (3): 271–282. doi:10.2307/2412495. JSTOR 2412495.
- Farris, J. S (1977). "Phylogenetic Analysis Under Dollo's Law". Systematic Biology. 26: 77–88. doi:10.1093/sysbio/26.1.77.
- Nelson, G (1979). "Cladistic Analysis and Synthesis: Principles and Definitions, with a Historical Note on Adanson's Familles Des Plantes (1763-1764)". Systematic Biology. 28: 1–21. doi:10.1093/sysbio/28.1.1.
- Gordon, A. D (1979). "A Measure of the Agreement between Rankings". Biometrika. 66 (1): 7–15. doi:10.1093/biomet/66.1.7. JSTOR 2335236.
- Efron B. (1979). Bootstrap methods: another look at the jackknife. Ann. Stat. 7: 1–26.
- Margush, T; McMorris, F (1981). "Consensus-trees". Bulletin of Mathematical Biology. 43 (2): 239. doi:10.1016/S0092-8240(81)90019-7.
- Sokal, Robert R; Rohlf, F. James (1981). "Taxonomic Congruence in the Leptopodomorpha Re-Examined". Systematic Zoology. 30 (3): 309. doi:10.2307/2413252. JSTOR 2413252.
- Felsenstein, Joseph (1981). "Evolutionary trees from DNA sequences: A maximum likelihood approach". Journal of Molecular Evolution. 17 (6): 368–76. Bibcode:1981JMolE..17..368F. doi:10.1007/BF01734359. PMID 7288891. S2CID 8024924.
- Hendy, M.D; Penny, David (1982). "Branch and bound algorithms to determine minimal evolutionary trees". Mathematical Biosciences. 59 (2): 277. doi:10.1016/0025-5564(82)90027-X.
- Lipscomb, Diana (1985). "The Eukaryotic Kingdoms". Cladistics. 1 (2): 127–40. doi:10.1111/j.1096-0031.1985.tb00417.x. PMID 34965673. S2CID 84151309.
- Felsenstein, J (1985). "Confidence limits on phylogenies: an approach using the bootstrap". Evolution. 39 (4): 783–791. doi:10.2307/2408678. JSTOR 2408678. PMID 28561359.
- Lanyon, S. M (1985). "Detecting Internal Inconsistencies in Distance Data". Systematic Biology. 34 (4): 397–403. CiteSeerX 10.1.1.1000.3956. doi:10.1093/sysbio/34.4.397.
- Saitou, N.; Nei, M. (1987). "The neighbor-joining method: A new method for reconstructing phylogenetic trees". Molecular Biology and Evolution. 4 (4): 406–25. doi:10.1093/oxfordjournals.molbev.a040454. PMID 3447015.
- Bremer, Kåre (1988). "The Limits of Amino Acid Sequence Data in Angiosperm Phylogenetic Reconstruction". Evolution. 42 (4): 795–803. doi:10.1111/j.1558-5646.1988.tb02497.x. PMID 28563878. S2CID 13647124.
- Farris, James S (1989). "The Retention Index and the Rescaled Consistency Index". Cladistics. 5 (4): 417–419. doi:10.1111/j.1096-0031.1989.tb00573.x. PMID 34933481. S2CID 84287895.
- Archie, James W (1989). "Homoplasy Excess Ratios: New Indices for Measuring Levels of Homoplasy in Phylogenetic Systematics and a Critique of the Consistency Index". Systematic Zoology. 38 (3): 253–269. doi:10.2307/2992286. JSTOR 2992286.
- Bremer, Kåre (1990). "Combinable Component Consensus". Cladistics. 6 (4): 369–372. doi:10.1111/j.1096-0031.1990.tb00551.x. PMID 34933485. S2CID 84151348.
- D. L. Swofford and G. J. Olsen. 1990. Phylogeny reconstruction. In D. M. Hillis and G. Moritz (eds.), Molecular Systematics, pages 411–501. Sinauer Associates, Sunderland, Mass.
- Goloboff, Pablo A (1991). "Homoplasy and the Choice Among Cladograms". Cladistics. 7 (3): 215–232. doi:10.1111/j.1096-0031.1991.tb00035.x. PMID 34933469. S2CID 85418697.
- Goloboff, Pablo A (1991). "Random Data, Homoplasy and Information". Cladistics. 7 (4): 395–406. doi:10.1111/j.1096-0031.1991.tb00046.x. S2CID 85132346.
- Goloboff, Pablo A (1993). "Estimating Character Weights During Tree Search". Cladistics. 9 (1): 83–91. doi:10.1111/j.1096-0031.1993.tb00209.x. PMID 34929936. S2CID 84231334.
- Wilkinson, M (1994). "Common Cladistic Information and its Consensus Representation: Reduced Adams and Reduced Cladistic Consensus Trees and Profiles". Systematic Biology. 43 (3): 343–368. doi:10.1093/sysbio/43.3.343.
- Wilkinson, Mark (1995). "More on Reduced Consensus Methods". Systematic Biology. 44 (3): 435–439. doi:10.2307/2413604. JSTOR 2413604.
- Li, Shuying; Pearl, Dennis K; Doss, Hani (2000). "Phylogenetic Tree Construction Using Markov Chain Monte Carlo". Journal of the American Statistical Association. 95 (450): 493. CiteSeerX 10.1.1.40.4461. doi:10.1080/01621459.2000.10474227. JSTOR 2669394. S2CID 122459537.
- Mau, Bob; Newton, Michael A; Larget, Bret (1999). "Bayesian Phylogenetic Inference via Markov Chain Monte Carlo Methods". Biometrics. 55 (1): 1–12. CiteSeerX 10.1.1.139.498. doi:10.1111/j.0006-341X.1999.00001.x. JSTOR 2533889. PMID 11318142. S2CID 932887.
- Rannala, Bruce; Yang, Ziheng (1996). "Probability distribution of molecular evolutionary trees: A new method of phylogenetic inference". Journal of Molecular Evolution. 43 (3): 304–11. Bibcode:1996JMolE..43..304R. doi:10.1007/BF02338839. PMID 8703097. S2CID 8269826.
- Goloboff, P (2003). "Improvements to resampling measures of group support". Cladistics. 19 (4): 324–32. doi:10.1111/j.1096-0031.2003.tb00376.x. hdl:11336/101057. S2CID 55516104.
- Li, M.; Chen, X.; Li, X.; Ma, B.; Vitanyi, P.M.B. (December 2004). "The Similarity Metric". IEEE Transactions on Information Theory. 50 (12): 3250–3264. doi:10.1109/TIT.2004.838101. S2CID 221927.
- Cilibrasi, R.; Vitanyi, P.M.B. (April 2005). "Clustering by Compression". IEEE Transactions on Information Theory. 51 (4): 1523–1545. arXiv:cs/0312044. doi:10.1109/TIT.2005.844059. S2CID 911.
- Pagel, Mark (2017). "Darwinian perspectives on the evolution of human languages". Psychonomic Bulletin & Review. 24 (1): 151–157. doi:10.3758/s13423-016-1072-z. ISSN 1069-9384. PMC 5325856. PMID 27368626.
- Heggarty, Paul (2006). "Interdisciplinary Indiscipline? Can Phylogenetic Methods Meaningfully Be Applied to Language Data — and to Dating Language?" (PDF). In Peter Forster; Colin Renfrew (eds.). Phylogenetic Methods and the Prehistory of Languages. McDonald Institute Monographs. McDonald Institute for Archaeological Research. Archived from the original (PDF) on 28 January 2021. Retrieved 19 January 2021.
- Bowern, Claire (14 January 2018). "Computational Phylogenetics". Annual Review of Linguistics. 4 (1): 281–296. doi:10.1146/annurev-linguistics-011516-034142. ISSN 2333-9683.
- Retzlaff, Nancy; Stadler, Peter F. (2018). "Phylogenetics beyond biology". Theory in Biosciences. 137 (2): 133–143. doi:10.1007/s12064-018-0264-7. ISSN 1431-7613. PMC 6208858. PMID 29931521.
- Hoffmann, Konstantin; Bouckaert, Remco; Greenhill, Simon J; Kühnert, Denise (25 November 2021). "Bayesian phylogenetic analysis of linguistic data using BEAST". Journal of Language Evolution. 6 (2): 119–135. doi:10.1093/jole/lzab005. ISSN 2058-458X.
- Spencer, Matthew; Davidson, Elizabeth A; Barbrook, Adrian C; Howe, Christopher J (21 April 2004). "Phylogenetics of artificial manuscripts". Journal of Theoretical Biology. 227 (4): 503–511. Bibcode:2004JThBi.227..503S. doi:10.1016/j.jtbi.2003.11.022. ISSN 0022-5193. PMID 15038985.
- "Phylogenetics - an overview | ScienceDirect Topics". www.sciencedirect.com. Retrieved 28 April 2023.
- Colijn, Gardy, Caroline, Jennifer (9 June 2014). ""Phylogenetic Tree Shapes Resolve Disease Transmission Patterns." Evolution, Medicine, and Public Health, U.S. National Library of Medicine". Evolution, Medicine, and Public Health. 2014 (1): 96–108. doi:10.1093/emph/eou018. PMC 4097963. PMID 24916411.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
Bibliography
- Schuh, Randall T.; Brower, Andrew V.Z. (2009). Biological Systematics: principles and applications (2nd ed.). Ithaca: Comstock Pub. Associates/Cornell University Press. ISBN 978-0-8014-4799-0. OCLC 312728177.
- Forster, Peter; Renfrew, Colin, eds. (2006). Phylogenetic Methods and the Prehistory of Languages. McDonald Institute Press, University of Cambridge. ISBN 978-1-902937-33-5. OCLC 69733654.
- Baum, David A.; Smith, Stacey D. (2013). Tree Thinking: an introduction to phylogenetic biology. Greenwood Village, CO: Roberts and Company. ISBN 978-1-936221-16-5. OCLC 767565978.
- Stuessy, Tod F. (2009). Plant Taxonomy: The Systematic Evaluation of Comparative Data. Columbia University Press. ISBN 978-0-231-14712-5.
External links
 The dictionary definition of phylogenetics at Wiktionary
The dictionary definition of phylogenetics at Wiktionary