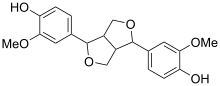
Pinoresinol
Pinoresinol is a tetrahydrofuran lignan[1] found in Styrax sp.,[2] Forsythia suspensa, and in Forsythia koreana.[3][4] It is also found in the caterpillar of the cabbage butterfly, Pieris rapae where it serves as a defence against ants.[5]
 | |
| Names | |
|---|---|
| IUPAC names
(+) form:
| |
| Other names
(+)-Pinoresinol (-)-Pinoresinol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C20H22O6 | |
| Molar mass | 358.38 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
In food, it is found in sesame seed, in Brassica vegetables[6] and in olive oil.[7] Pinoresinol has also been found to be toxic to larvae of the milkweed bug Oncopeltus fasciatus and of the haematophagous insect Rhodnius prolixus, which is a vector of chagas disease.[8]
Currently, pinoresinol is isolated from plants with low efficiency and low yield.[9]
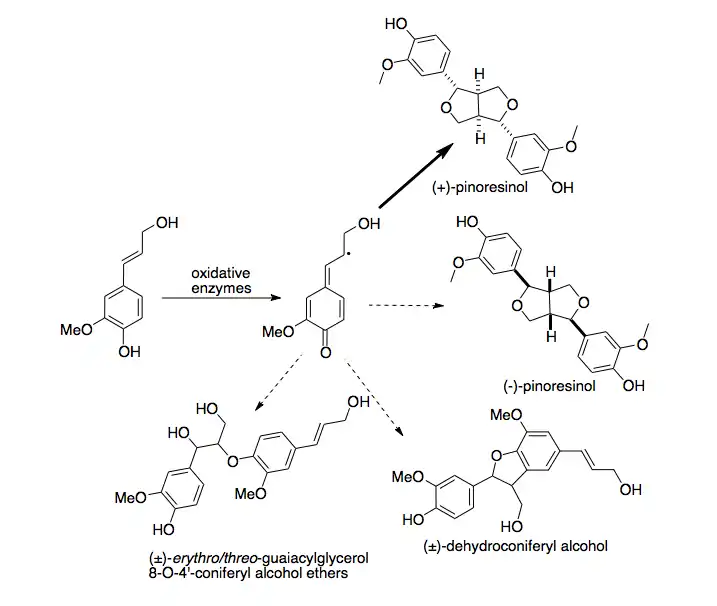
Biosynthesis
A first dirigent protein was discovered in Forsythia intermedia. This protein has been found to direct the stereoselective biosynthesis of (+)-pinoresinol from coniferyl alcohol monomers.[10] Recently, a second, enantiocomplementary dirigent protein was identified in Arabidopsis thaliana, which directs enantioselective synthesis of (-)-pinoresinol.[11]
-Pinoresinol_Biosynthesis.svg.png.webp)
Pharmacology
Pinoresinol inhibits the enzyme α-glucosidase in vitro and may therefore act as a hypoglycemic agent.[12] A study involving extra virgin olive oil showed that pinoresinol possess in vitro chemoprevention properties. Increased apoptosis and cellular arrest at the G2/M stage in p53-proficient cells occurred.[13] Pinoresinol of olive oil decreases vitamin D intestinal absorption.[14]
Metabolism into enterolignans
Pinoresinol, along with other plant lignans, are converted into enterolignans by intestinal microflora in the human body.[15]
See also
References
- Páska, Csilla; Innocenti, Gabbriella; Ferlin, Mariagrazia; Kunvári, Mónika; László, Miklós (January 2002). "Pinoresinol from Ipomoea Cairica Cell Cultures". Natural Product Letters. 16 (5): 359–363. doi:10.1080/1057530290033123. ISSN 1057-5634. PMID 12434993. S2CID 13015216.
- Pastrorova et al. (1997)
- Jung, Hyo Won; Mahesh, Ramalingam; Lee, Jong Gu; Lee, Seung Ho; Kim, Young Shik; Park, Yong-Ki (August 2010). "Pinoresinol from the fruits of Forsythia koreana inhibits inflammatory responses in LPS-activated microglia". Neuroscience Letters. 480 (3): 215–220. doi:10.1016/j.neulet.2010.06.043. ISSN 0304-3940. PMID 20600612. S2CID 41511857.
- Davin, Laurence B.; Bedgar, Diana L.; Katayama, Takeshi; Lewis, Norman G. (1992). "On the stereoselective synthesis of (+)-pinoresinol in Forsythia suspensa from its achiral precursor, coniferyl alcohol". Phytochemistry. 31 (11): 3869–74. doi:10.1016/S0031-9422(00)97544-7. PMID 11536515.
- Schroeder, F. C.; Del Campo, M. L.; Grant, J. B.; Weibel, D. B.; Smedley, S. R.; Bolton, K. L.; Meinwald, J.; Eisner, T. (2006). "Pinoresinol: A lignol of plant origin serving for defense in a caterpillar". Proceedings of the National Academy of Sciences. 103 (42): 15497–501. Bibcode:2006PNAS..10315497S. doi:10.1073/pnas.0605921103. PMC 1622851. PMID 17030818.
- Milder, Ivon E. J.; Arts, Ilja C. W.; Putte, Betty van de; Venema, Dini P.; Hollman, Peter C. H. (2007). "Lignan contents of Dutch plant foods: A database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol". British Journal of Nutrition. 93 (3): 393–402. doi:10.1079/BJN20051371. PMID 15877880.
- Owen, R.W; Giacosa, A; Hull, W.E; Haubner, R; Spiegelhalder, B; Bartsch, H (2000). "The antioxidant/anticancer potential of phenolic compounds isolated from olive oil". European Journal of Cancer. 36 (10): 1235–47. doi:10.1016/S0959-8049(00)00103-9. PMID 10882862.
- Cabral, M.M.O; Kelecom, A; Garcia, E.S (December 1999). "Effects of the lignan, pinoresinol on the moulting cycle of the bloodsucking bug Rhodnius prolixus and of the milkweed bug Oncopeltus fasciatus". Fitoterapia. 70 (6): 561–567. doi:10.1016/s0367-326x(99)00089-1. ISSN 0367-326X.
- Lv, Yongkun; Cheng, Xiaozhong; Du, Guocheng; Zhou, Jingwen; Chen, Jian (2017-05-12). "Engineering of an H2 O2 auto-scavenging in vivo cascade for pinoresinol production". Biotechnology and Bioengineering. 114 (9): 2066–2074. doi:10.1002/bit.26319. ISSN 0006-3592. PMID 28436004. S2CID 3289052.
- Davin LB, Wang HB, Crowell AL, et al. (1997). "Stereoselective bimolecular phenoxy radical coupling by an auxiliary (dirigent) protein without an active center". Science. 275 (5298): 362–6. doi:10.1126/science.275.5298.362. PMID 8994027. S2CID 41957412.
- Pickel B, Constantin MA, Pfannsteil J, Conrad J, Beifuss U, Schaffer A (March 2007). "An Enantiocomplementary Dirigent Protein for the Enantioselective Laccase-Catalyzed Oxidative Coupling of Phenols". Angewandte Chemie. 53 (4): 273–284. doi:10.1007/s10086-007-0892-x. S2CID 195313754.
- Wikul, A; Damsud, T; Kataoka, K; Phuwapraisirisan, P (2012). "(+)-Pinoresinol is a putative hypoglycemic agent in defatted sesame (Sesamum indicum) seeds though inhibiting α-glucosidase". Bioorganic & Medicinal Chemistry Letters. 22 (16): 5215–7. doi:10.1016/j.bmcl.2012.06.068. PMID 22818971.
- Fini, L; Hotchkiss, E; Fogliano, V; Graziani, G; Romano, M; De Vol, EB; Qin, H; Selgrad, M; et al. (2008). "Chemopreventive properties of pinoresinol-rich olive oil involve a selective activation of the ATM-p53 cascade in colon cancer cell lines". Carcinogenesis. 29 (1): 139–46. doi:10.1093/carcin/bgm255. PMID 17999988.
- Goncalves, Aurélie; Margier, Marielle; Tagliaferri, Camille; Lebecque, Patrice; Georgé, Stéphane; Wittrant, Yohann; Coxam, Véronique; Amiot, Marie-Josèphe; Reboul, Emmanuelle (September 2016). "Pinoresinol of olive oil decreases vitamin D intestinal absorption". Food Chemistry. 206: 234–238. doi:10.1016/j.foodchem.2016.03.048. ISSN 0308-8146. PMID 27041321.
- Milder, IE; Arts, IC; Van De Putte, B; Venema, DP; Hollman, PC (2005). "Lignan contents of Dutch plant foods: A database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol". The British Journal of Nutrition. 93 (3): 393–402. doi:10.1079/BJN20051371. PMID 15877880.