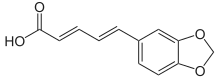
Piperic acid
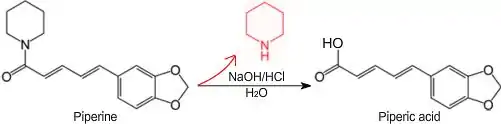
Piperic acid is a chemical often obtained by the base-hydrolysis of the alkaloid piperine[1] from black pepper,[2] followed by acidification of the corresponding salt. Piperic acid is an intermediate in the synthesis of other compounds such as piperonal, and as-such may be used to produce fragrances, perfumes flavorants and drugs as well as other useful compounds.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(2E,4E)-5-(2H-1,3-Benzodioxol-5-yl)penta-2,4-dienoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| MeSH | C017637 |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C12H10O4 | |
| Molar mass | 218.208 g·mol−1 |
| Boiling point | decomposes |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Preparation
Piperic acid can be prepared from the commercially-available alkaloid piperine, a cyclic amide containing a piperidine group, by reacting it with a hydroxide such as potassium hydroxide, then acidifying the formed piperate salt with hydrochloric acid or another acid. The toxic compound piperidine is given off during the base-hydrolysis of piperine and as-such, safety precautions should be taken.
Reactions
Reaction of piperic acid with strong oxidizers such as potassium permanganate or ozone, or a halogen such as bromine followed by sodium hydroxide causes oxidative cleavage of the double-bonds, yielding piperonal and piperonylic acid.[3] Piperonal has many uses in industry and is itself a precursor to a good subsection of other chemicals. On reduction with sodium amalgam piperic acid forms α- and β-dihydropiperic acid, C12H12O4, and the latter can take up two further atoms of hydrogen to produce tetrahydropiperic acid.
References
- Paul M. Dewick. (2009). Medicinal natural products : a biosynthetic approach. Chichester: A John Wiley & Sons. p. 327. ISBN 978-0-470-74167-2.
- E. Gildemeister. The Volatile Oils. Vol. 1.
- US 5095128, "Preparation process for piperonal"