Plasmid-mediated resistance
Plasmid-mediated resistance is the transfer of antibiotic resistance genes which are carried on plasmids.[1] Plasmids possess mechanisms that ensure their independent replication as well as those that regulate their replication number and guarantee stable inheritance during cell division. By the conjugation process, they can stimulate lateral transfer between bacteria from various genera and kingdoms.[2] Numerous plasmids contain addiction-inducing systems that are typically based on toxin-antitoxin factors and capable of killing daughter cells that don't inherit the plasmid during cell division.[3] Plasmids often carry multiple antibiotic resistance genes, contributing to the spread of multidrug-resistance (MDR).[4] Antibiotic resistance mediated by MDR plasmids severely limits the treatment options for the infections caused by Gram-negative bacteria, especially family Enterobacteriaceae.[5] The global spread of MDR plasmids has been enhanced by selective pressure from antimicrobial medications used in medical facilities and when raising animals for food.[6]
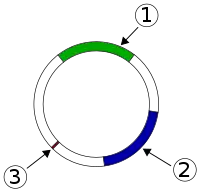
Properties of resistance plasmids
Resistance plasmids by definition carry one or more antibiotic resistance genes.[7] They are frequently accompanied by the genes encoding virulence determinants,[8] specific enzymes or resistance to toxic heavy metals.[9] Multiple resistance genes are commonly arranged in the resistance cassettes.[7] The antibiotic resistance genes found on the plasmids confer resistance to most of the antibiotic classes used nowadays, for example, beta-lactams, fluoroquinolones and aminoglycosides.[10]
It is very common for the resistance genes or entire resistance cassettes to be re-arranged on the same plasmid or be moved to a different plasmid or chromosome by means of recombination systems. Examples of such systems include integrons, transposons, and ISCR-promoted gene mobilization.[7]
Most of the resistance plasmids are conjugative, meaning that they encode all the needed components for the transfer of the plasmid to another bacterium,[11] and that isn't present in mobilizable plasmids. According to that, Mobilizable plasmids are smaller in size (usually < 10 kb) while conjugative plasmids are largger (usually > 30 kb) due to the considerable size of DNA required to encode the conjugation mechanisms that allow for cell-to-cell conjugation.[7]
R-factor
R-factors are also called a resistance factors or resistance plasmid. They are tiny, circular DNA elements that are self-replicating,[12] that contain antibiotic resistance genes.[13] They were first found in Japan in 1959 when it was discovered that some Shigella strains had developed resistance to a number of antibiotics used to treat a dysentery epidemic. Shigella is a genus of Gram-negative, aerobic, non-spore-forming, non-motile, rod-shaped bacteria.[12] Resistance genes are ones that give rise to proteins that modify the antibiotic or pump it out. They are different from mutations that give bacteria resistance to antibiotics by preventing the antibiotic from getting in or changing the shape of the target protein.[14] R-factors have been known to contain up to ten resistance genes. They can also spread easily as they contain genes for constructing pili, which allow them to transfer the R-factor to other bacteria.[15] R-factors have contributed to the growing antibiotic resistance crisis because they quickly spread resistance genes among bacteria.[16] The R factor by itself cannot be transmitted.[12]
Structure of Resistance Plasmids
The majority of the R-RTF (Resistance Transfer Factor) molecules are found in the resistance plasmid, which can be conceptualized as a circular piece of DNA with a length of 80 to 95 kb.[12] This plasmid shares many genes with the F factor and is largely homologous to it.[17] Additionally, it has a fin 0 gene that inhibits the transfer operon's functionality. The size and number of drug resistance genes in each R factor varies.The RTF is bigger than the R determinant. An IS 1 element separates the RTF and R determinant on either side before they combine into a single unit.The IS 1 components simplify it for R determinants to be transferred between different R-RTF unit types.[12]
Functions of Resistance Plasmids
Transmission
Bacteria containing F-factors (said to be "F+") have the capability for horizontal gene transfer; they can construct a sex pilus, which emerges from the donor bacterium and ensnares the recipient bacterium, draws it in,[18] and eventually triggers the formation of a mating bridge, merging the cytoplasms of two bacteria via a controlled pore.[19] This pore allows the transfer of genetic material, such as a plasmid. Conjugation allows two bacteria, not necessarily from the same species, to transfer genetic material one way.[20] Since many R-factors contain F-plasmids, antibiotic resistance can be easily spread among a population of bacteria.[21] Also, R-factors can be taken up by "DNA pumps" in their membranes via transformation,[22] or less commonly through viral mediated transduction,[23] or via bacteriophage, although conjugation is the most common means of antibiotic resistance spread. They contain the gene called RTF (Resistance transfer factor).
Enterobacteriaceae
it is a family of Gram-negative rod-shaped (bacilli) bacteria, the pathogenic bacteria that are most frequently found in the environment and clinical cases, as a result, they are significantly impacted by the use of antibiotics in agriculture, the ecosystem, or the treatment of diseases.[24] In Enterobacteriaceae, 28 different plasmid types can be identified by PCR-based replicon typing (PBRT).The plasmids that have been frequently reported [IncF, IncI, IncA/C, IncL (previously designated IncL/M), IncN, and IncH] contain a broad variety of resistance genes.[25]
Members of family Enterobacteriaceae, for example, Escherichia coli or Klebsiella pneumoniae pose the biggest threat regarding plasmid-mediated resistance in hospital- and community-acquired infections.[5]
Beta-lactam resistance
B-lactamases are antibiotic-hydrolyzing enzymes that typically cause resistance to b-lactam antibiotics. These enzymes are prevalent in Streptomyces, and together with related enzymes discovered in pathogenic and non-pathogenic bacteria, they form the protein family known as the "b-lactamase superfamily".[14] it is hypothesized that b-lactamases also serve a double purpose, such as housekeeping and antibiotic resistance.[26]
Both narrow spectrum beta-lactamases (e.g. penicillinases) and extended spectrum beta-lactamases (ESBL) are common for resistance plasmids in Enterobacteriaceae. Often multiple beta-lactamase genes are found on the same plasmid hydrolyzing a wide spectrum of beta-lactam antibiotics.[5]
Extended spectrum beta-lactamases (ESBL)
ESBL enzymes can hydrolyze all beta-lactam antibiotics, including cephalosporins, except for the carpabepenems. The first clinically observed ESBL enzymes were mutated versions of the narrow spectrum beta-lactamases, like TEM and SHV. Other ESBL enzymes originate outside of family Enterobacteriaceae, but have been spreading as well.[5]
In addition, since the plasmids that carry ESBL genes also commonly encode resistance determinants for many other antibiotics, ESBL strains are often resistant to many non-beta-lactam antibiotics as well,[27] leaving very few options for the treatment.
Carbapenemases
Carbapenemases represent type of ESBL which are able to hydrolyze carbapenem antibiotics that are considered as the last-resort treatment for ESBL-producing bacteria. KPC, NDM-1, VIM and OXA-48 carbapenemases have been increasingly reported worldwide as causes of hospital-acquired infections.[5]
Quinolone resistance
Several studies have shown that fluoroquinolone resistance has enhanced worldwide, especially in Enterobacteriaceae members. QnrA was the first known plasmid-mediated gene associated in quinolone resistance.[28]Quinolone resistance genes are frequently located on the same plasmid as the ESBL genes.[29] The proteins known as QnrS, QnrB, QnrC, and QnrD are four others that are similar. Numerous variants have been found for qnrA, qnrS, and qnrB, and they are distinguished by sequential numbers.[30] The qnr genes can be discovered in integrons and transposons on MDR plasmids of various incompatibility groups, which could carry a number of resistance-related molecules, such as carbapenemases and ESBLs.[31] Examples of resistance mechanisms include different Qnr proteins, aminoglycose acetyltransferase aac(6')-Ib-cr that is able to hydrolyze ciprofloxacin and norfloxacin, as well as efflux transporters OqxAB and QepA.[5]
Aminoglycoside resistance
xResistance to aminoglycosides in Gram-negative pathogens is primarily caused by enzymes that acetylate, adenylate, or phosphorylate the medication.[32] On mobile elements, such as plasmids, are the genes that encode these enzymes.[33]Aminoglycoside resistance genes are also commonly found together with ESBL genes. Resistance to aminoglycosides is conferred via numerous aminoglycoside-modifying enzymes and 16S rRNA methyltransferases.[5] Resistance to aminoglycosides is conferred via numerous mechanisms:
- aminoglycoside-modifying enzymes and inactivation of the aminoglycosides, which is frequently seen in both gram-positive and gram-negative bacteria and is induced by nucleotidyltransferases, phosphotransferases, or aminoglycoside acetyltransferases.
- reduced permeability.
- enhanced efflux.
- variations to the 30S ribosomal subunit that prevent aminoglycosides from binding to it.[34]
small RNAs
Study investigating physiological effect of pHK01 plasmid in host E.coli J53 found that the plasmid reduced bacterial motility and conferred resistance to beta-lactams. The pHK01 produced plasmid-encoded small RNAs and mediated expression of host sRNAs. These sRNAs were antisense to genes involved in replication, conjugate transfer and plasmid stabilisation : AS-repA3 (CopA), AS-traI, AS-finO, AS-traG, AS-pc02 . The over-expression of one of the plasmid-encoded antisense sRNAs: AS-traI shortened t lalog phase of host growth.[35]
References
- San Millan A (December 2018). "Evolution of Plasmid-Mediated Antibiotic Resistance in the Clinical Context". Trends in Microbiology. 26 (12): 978–985. doi:10.1016/j.tim.2018.06.007. PMID 30049587.
- "conjugation (prokaryotes) | Learn Science at Scitable". www.nature.com. Retrieved 6 January 2023.
- Tsang J (May 2017). "Bacterial plasmid addiction systems and their implications for antibiotic drug development". Postdoc Journal. 5 (5): 3–9. PMC 5542005. PMID 28781980.
- Nikaido H (1 June 2009). "Multidrug resistance in bacteria". Annual Review of Biochemistry. 78 (1): 119–146. doi:10.1146/annurev.biochem.78.082907.145923. PMC 2839888. PMID 19231985.
- Schultsz C, Geerlings S (January 2012). "Plasmid-mediated resistance in Enterobacteriaceae: changing landscape and implications for therapy". Drugs. 72 (1): 1–16. doi:10.2165/11597960-000000000-00000. PMID 22191792. S2CID 42306704.
- Mathers AJ, Peirano G, Pitout JD (July 2015). "The role of epidemic resistance plasmids and international high-risk clones in the spread of multidrug-resistant Enterobacteriaceae". Clinical Microbiology Reviews. 28 (3): 565–591. doi:10.1128/CMR.00116-14. PMC 4405625. PMID 25926236.
- Bennett PM (March 2008). "Plasmid encoded antibiotic resistance: acquisition and transfer of antibiotic resistance genes in bacteria". British Journal of Pharmacology. 153 (Suppl 1): S347–S357. doi:10.1038/sj.bjp.0707607. PMC 2268074. PMID 18193080.
- Darmancier H, Domingues CP, Rebelo JS, Amaro A, Dionísio F, Pothier J, et al. (May 2022). "Are Virulence and Antibiotic Resistance Genes Linked? A Comprehensive Analysis of Bacterial Chromosomes and Plasmids". Antibiotics. 11 (6): 706. doi:10.3390/antibiotics11060706. PMC 9220345. PMID 35740113.
- Sevim A, Sevim E (2015). "Plasmid Mediated Antibiotic and Heavy Metal Resistance in Bacillus Strains Isolated from Soils in Rize, Turkey". Suleyman Demirel University Journal of Natural and Applied Science. 19 (2): 133–141 – via Süleyman Demirel University science Institute.
- McMillan EA, Gupta SK, Williams LE, Jové T, Hiott LM, Woodley TA, et al. (2019). "Antimicrobial Resistance Genes, Cassettes, and Plasmids Present in Salmonella enterica Associated With United States Food Animals". Frontiers in Microbiology. 10: 832. doi:10.3389/fmicb.2019.00832. PMC 6479191. PMID 31057528.
- Zatyka M, Thomas CM (1998). "Control of genes for conjugative transfer of plasmids and other mobile elements". FEMS Microbiology Reviews. 21 (4): 291–319. doi:10.1111/j.1574-6976.1998.tb00355.x. PMID 25481925.
- "R-Factor - Structure and Functions of Resistance Factors or Plasmids". BYJUS. Retrieved 11 January 2023.
- "R-Factor". VEDANTU. Retrieved 8 January 2023.
- Peterson E, Kaur P (2018). "Antibiotic Resistance Mechanisms in Bacteria: Relationships Between Resistance Determinants of Antibiotic Producers, Environmental Bacteria, and Clinical Pathogens". Frontiers in Microbiology. 9: 2928. doi:10.3389/fmicb.2018.02928. PMC 6283892. PMID 30555448.
- Tao S, Chen H, Li N, Wang T, Liang W (18 July 2022). "The Spread of Antibiotic Resistance Genes In Vivo Model". The Canadian Journal of Infectious Diseases & Medical Microbiology. 2022: 3348695. doi:10.1155/2022/3348695. PMC 9314185. PMID 35898691.
- Campbell N (2018). Biology A Global Approach (11th ed.). New York: Pearson. p. 633. ISBN 978-1-292-17043-5.
- Fernandez-Lopez R, de Toro M, Moncalian G, Garcillan-Barcia MP, de la Cruz F (2016). "Comparative Genomics of the Conjugation Region of F-like Plasmids: Five Shades of F". Frontiers in Molecular Biosciences. 3: 71. doi:10.3389/fmolb.2016.00071. PMC 5102898. PMID 27891505.
- Bragagnolo N, Rodriguez C, Samari-Kermani N, Fours A, Korouzhdehi M, Lysenko R, Audette GF (September 2020). "Protein Dynamics in F-like Bacterial Conjugation". Biomedicines. 8 (9): 362. doi:10.3390/biomedicines8090362. PMC 7555446. PMID 32961700.
- Virolle C, Goldlust K, Djermoun S, Bigot S, Lesterlin C (October 2020). "Plasmid Transfer by Conjugation in Gram-Negative Bacteria: From the Cellular to the Community Level". Genes. 11 (11): 1239. doi:10.3390/genes11111239. PMC 7690428. PMID 33105635.
- "Prokaryotic Cell Structure: Pili". Archived from the original on 7 December 2016. Retrieved 19 January 2017.
- Helinski DR (December 2022). Kaper JB (ed.). "A Brief History of Plasmids". EcoSal Plus. 10 (1): eESP00282021. doi:10.1128/ecosalplus.ESP-0028-2021. PMID 35373578. S2CID 254686832.
- Burton B, Dubnau D (July 2010). "Membrane-associated DNA transport machines". Cold Spring Harbor Perspectives in Biology. 2 (7): a000406. doi:10.1101/cshperspect.a000406. PMC 2890206. PMID 20573715.
- Nayerossadat N, Maedeh T, Ali PA (2012). "Viral and nonviral delivery systems for gene delivery". Advanced Biomedical Research. 1: 27. doi:10.4103/2277-9175.98152. PMC 3507026. PMID 23210086.
- Dahal P (20 May 2022). "Enterobacteriaceae- Definition, Characteristics, Identification". Microbe Notes. Retrieved 9 January 2023.
- Rozwandowicz M, Brouwer MS, Fischer J, Wagenaar JA, Gonzalez-Zorn B, Guerra B, et al. (May 2018). "Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae". The Journal of Antimicrobial Chemotherapy. 73 (5): 1121–1137. doi:10.1093/jac/dkx488. PMID 29370371.
- Baquero F, Bouza E, Gutiérrez-Fuentes J, Coque TM (January 2018). "Ecology and Evolution of Chromosomal Gene Transfer between Environmental Microorganisms and Pathogens". Microbiology Spectrum. 6 (1): 6.1.06. doi:10.1128/microbiolspec.MTBP-0006-2016. PMID 29350130.
- Hussain T, Jamal M, Nighat F, Andleeb S (2014). "Broad spectrum antibiotics and resistance in non-target bacteria: an example from tetracycline". Journal of Pure and Applied Microbiology. 8 (4): 2667–2671.
- Poirel L, Rodriguez-Martinez JM, Mammeri H, Liard A, Nordmann P (August 2005). "Origin of plasmid-mediated quinolone resistance determinant QnrA". Antimicrobial Agents and Chemotherapy. 49 (8): 3523–3525. doi:10.1128/AAC.49.8.3523-3525.2005. PMC 1196254. PMID 16048974.
- Salah FD, Soubeiga ST, Ouattara AK, Sadji AY, Metuor-Dabire A, Obiri-Yeboah D, et al. (18 June 2019). "Distribution of quinolone resistance gene (qnr) in ESBL-producing Escherichia coli and Klebsiella spp. in Lomé, Togo". Antimicrobial Resistance and Infection Control. 8 (1): 104. doi:10.1186/s13756-019-0552-0. PMC 6582466. PMID 31244995.
- Azeez DA, Findik D, Hatice TÜ, Arslan U (2018). "Plasmid-mediated fluoroquinolone resistance in clinical isolates of Escherichia coli in Konya, Turkey". Cukurova Medical Journal. 43 (2): 295–300. doi:10.17826/cumj.341637. S2CID 49553107.
- Strahilevitz J, Jacoby GA, Hooper DC, Robicsek A (October 2009). "Plasmid-mediated quinolone resistance: a multifaceted threat". Clinical Microbiology Reviews. 22 (4): 664–689. doi:10.1128/CMR.00016-09. PMC 2772364. PMID 19822894.
- Ramirez MS, Tolmasky ME (December 2017). "Amikacin: Uses, Resistance, and Prospects for Inhibition". Molecules. 22 (12): 2267. doi:10.3390/molecules22122267. PMC 5889950. PMID 29257114.
- Belaynehe KM, Shin SW, Hong-Tae P, Yoo HS (August 2017). "Occurrence of aminoglycoside-modifying enzymes among isolates of Escherichia coli exhibiting high levels of aminoglycoside resistance isolated from Korean cattle farms". FEMS Microbiology Letters. 364 (14). doi:10.1093/femsle/fnx129. PMID 28637330.
- Doi Y, Wachino JI, Arakawa Y (June 2016). "Aminoglycoside Resistance: The Emergence of Acquired 16S Ribosomal RNA Methyltransferases". Infectious Disease Clinics of North America. Antibiotic Resistance: Challenges and Opportunities. 30 (2): 523–537. doi:10.1016/j.idc.2016.02.011. PMC 4878400. PMID 27208771.
- Jiang X, Liu X, Law CO, Wang Y, Lo WU, Weng X, et al. (July 2017). "The CTX-M-14 plasmid pHK01 encodes novel small RNAs and influences host growth and motility". FEMS Microbiology Ecology. 93 (7). doi:10.1093/femsec/fix090. PMID 28854680.
Further reading
- Strahilevitz J, Jacoby GA, Hooper DC, Robicsek A (October 2009). "Plasmid-mediated quinolone resistance: a multifaceted threat". Clinical Microbiology Reviews. 22 (4): 664–689. doi:10.1128/CMR.00016-09. PMC 2772364. PMID 19822894.
- Nordmann P, Poirel L (September 2005). "Emergence of plasmid-mediated resistance to quinolones in Enterobacteriaceae". The Journal of Antimicrobial Chemotherapy. 56 (3): 463–469. doi:10.1093/jac/dki245. PMID 16020539.
- Oktem IM, Gulay Z, Bicmen M, Gur D (January 2008). "qnrA prevalence in extended-spectrum beta-lactamase-positive Enterobacteriaceae isolates from Turkey". Japanese Journal of Infectious Diseases. 61 (1): 13–17. doi:10.7883/yoken.JJID.2008.13. PMID 18219128. S2CID 24744915.
- Chen LP, Cai XW, Wang XR, Zhou XL, Wu DF, Xu XJ, Chen HC (October 2010). "Characterization of plasmid-mediated lincosamide resistance in a field isolate of Haemophilus parasuis". The Journal of Antimicrobial Chemotherapy. 65 (10): 2256–2258. doi:10.1093/jac/dkq304. PMID 20699244.