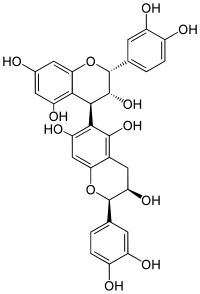
Procyanidin B5
Procyanidin B5 is a B type proanthocyanidin.
 | |
| Names | |
|---|---|
| IUPAC name
[(2R,3R,4S)-Flavan-3,3′,4′,5,7-pentol]-(4→6)-[(2R,3R)-flavan-3,3′,4′,5,7-pentol] | |
| Systematic IUPAC name
(2R,2′R,3R,3′R,4S)-2,2′-Bis(3,4-dihydroxyphenyl)-3,3′,4,4′-tetrahydro-2H,2′H-[4,6′-bi-1-benzopyran]-3,3′,5,5′,7,7′-hexol | |
| Other names
Procyanidin B5 | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C30H26O12 | |
| Molar mass | 578.52 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Procyanidin B5 is an epicatechin-(4β → 6)-epicatechin dimer.
Natural occurrences
It can be found in grape seeds,[1] in Hibiscus cannabinus (kenaf) root and bark,[2] and in black chokeberries (Aronia melanocarpa).[3]
- Presence in food
References
- Procyanidin dimers and trimers from grape seeds. Jorge M. Ricardo da Silva, Jacques Rigaud, Véronique Cheynier, Annie Cheminat and Michel Moutounet, Phytochemistry, Volume 30, Issue 4, 1991, Pages 1259-1264
- Dimeric proanthocyanidins of Hibiscus cannabinus. Fam Van Tkhin', B. Makhsudova and O. S. Otroshchenko, Chemistry of Natural Compounds, Volume 18, Number 3, pages 310-314, doi:10.1007/BF00580458
- Preparation of Dimeric Procyanidins B1, B2, B5, and B7 from a Polymeric Procyanidin Fraction of Black Chokeberry (Aronia melanocarpa). Tuba Esatbeyoglu and Peter Winterhalter, J. Agric. Food Chem., 2010, 58 (8), pages 5147–5153, doi:10.1021/jf904354n
- Isolation of dimeric, trimeric, tetrameric and pentameric procyanidins from unroasted cocoa beans (Theobroma cacao L.) using countercurrent chromatography. Esatbeyoglu T, Wray Vand Winterhalter P, Food Chem., 2015 Jul 15, number 179, pages 278-89, doi:10.1016/j.foodchem.2015.01.130
- Rapid Reversed Phase Ultra-Performance Liquid Chromatography Analysis of the Major Cocoa Polyphenols and Inter-relationships of Their Concentrations in Chocolate. Karen A. Cooper, Esther Campos-Gimenez, Diego Jimenez-Alvarez, Kornel Nagy, Jennifer L. Donovan and Gary Williamson, J. Agric. Food Chem., 2007, 55, pages 2841−2847, doi:10.1021/jf063277c
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.