Prodan (dye)
Prodan is a fluorescent dye (a naphthalene derivative) used as a membrane probe with environment-sensitive coloration,[6][7] as well as a non-covalently bonding probe for proteins.[7]
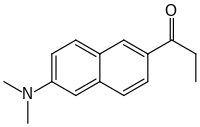 | |
| Names | |
|---|---|
| Preferred IUPAC name
1-[6-(Dimethylamino)naphthalen-2-yl]propan-1-one | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| 2723587 | |
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H17NO | |
| Molar mass | 227.307 g·mol−1 |
| Melting point | 137 °C (279 °F; 410 K)[3] |
| Hazards[4][5] | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
May cause skin/eye irritation |
| Safety data sheet (SDS) | SDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Prodan was proposed as a membrane dye by Weber and Farris in 1979. Since then, multiple derivatives have been introduced, such as lypophilic Laurdan (derivative of lauric acid) and thiol-reactive Badan (bromoacetic acid derivative) and Acrylodan.[7]
Being a push-pull dye, Prodan has a large excited-state dipole moment and consequently high sensibility to the polarity of its environment (solvent or cell membrane, including the physical state of surrounding phospholipids). Usually it is concentrated at the surface of the membrane, with some degree of penetration. Excited-state relaxation of prodan is sensitive to whether the linkage between phospholipid hydrocarbon tails and the glycerol backbone is of ether or ester type. Therefore, many studies exploited this sensitivity to explore coexisting lipid domains in dual-wavelength ratio measurements, to detect non-bilayer lipid phases, to map membrane structure changes.[7] However, Prodan presence itself is found to change the structure of membranes.[6]
In proteins, Prodan and Badan are quenched by non-covalent bonding with tryptophan and the oxidation of it by excited Prodan/Badan.[8]
Absorption of Prodan lies in the UV range (361 nm in methanol),[3] but two-photon excitation techniques have been successfully applied. Fluorescence wavelength is highly sensitive to the polarity of the environment. For example, the emission wavelength shifts from 380 nm in cyclohexane to 450 nm in DMFA[7] to 498 nm in methanol[3] to 520 nm in water.[7] Cyclic voltammetry shows a reversible reduction peak at —1.85 V in acetonitrile and a quasi-reversible one at —0.88 in aqueous buffer (pH 7.3) (vs. NHE). Contrariwise, excited-state reduction potential is +1.6 V in the acetonitrile and +0.6 V in water (vs. NHE).[8] Photostability of Prodan and Laurdan in low-polaritry environments is limited due to undergoing an intersystem crossing in the excited state, with subsequent reactions with triplet oxygen (this also diminishes its quantum yield, which is 0.95 in ethanol but only 0.03 in cyclohexane).[9]
References
- "Prodan (6-Propionyl-2-Dimethylaminonaphthalene)". Thermo Fisher Scientific. Retrieved 22 October 2019.
- "Prodan". PubChem. National Center for Biotechnology Information. Retrieved 22 October 2019.
- "N,N-Dimethyl-6-propionyl-2-naphthylamine". Merck. Sigma-Aldrich. Retrieved 22 October 2019.
- Safety Data Sheet. Life Technologies, Inc., Feb 6th 2015
- Safety Data Sheet. Sigma-Aldrich Co., 2018
- Suhaj, Adam; Le Marois, Alix; Williamson, David J.; Suhling, Klaus; Lorenz, Christian D.; Owen, Dylan M. (31 May 2018). "PRODAN differentially influences its local environment". Physical Chemistry Chemical Physics. 20 (23): 16060–16066. doi:10.1039/C8CP00543E. PMID 29850681. Retrieved 22 October 2019.
- The Molecular Probes™ Handbook: A Guide to Fluorescent Probes and Labeling Technologies (PDF) (11 ed.). Thermo Fisher Scientific. 2010. pp. 579–584.
- Pospíšil, Petr; Luxem, Katja E.; Ener, Maraia; Sýkora, Jan; Kocábová, Jana; Gray, Harry B.; Vlček, Jr., Antonín; Hof, Martin (28 August 2014). "Fluorescence Quenching of (Dimethylamino)naphthalene Dyes Badan and Prodan by Tryptophan in Cytochromes P450 and Micelles". Journal of Physical Chemistry B. 118 (34): 10085–10091. doi:10.1021/jp504625d. PMC 4148165. PMID 25079965.
- Niko, Yosuke; Didier, Pascal; Mely, Yves; Konishi, Gen-ichi; Klymchenko, Andrey S. (11 January 2016). "Bright and photostable push-pull pyrene dye visualizes lipid order variation between plasma and intracellular membranes". Scientific Reports. 6: 18870. doi:10.1038/srep18870. PMC 4707542. PMID 26750324.