Prodelphinidin
Prodelphinidin is a name for the polymeric tannins composed of gallocatechin.[1][2][3] It yields delphinidin during depolymerisation under oxidative conditions.
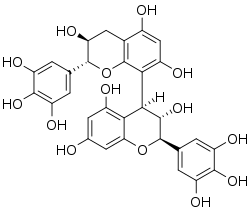
Natural occurrences
Prodelphinidins are one of the two sorts of tannins in grape (the other being procyanidins) being produced especially in the skin of the berry.[4]
Prodelphinidins can be found in Cistus salviifolius.[5] Gallocatechin-(4→8)-catechin (prodelphinidin B3), gallocatechin-(4→8)-gallocatechin and catechin-(4→8)-gallocatechin can be found in the pomegranate peels.[6] Prodelphinidin B-2 3'-O-gallate can be found in green tea leaves[7] and prodelphinidin B-2 3,3'-di-O-gallate can be found in Myrica rubra.[8]
Particular oligomeric prodelphinidins
Prodelphinidin B3 (gallocatechin-(4α→8)-catechin) and prodelphinidin B9 (epigallocatechin-(4α→8)-catechin) can be isolated in beer.[9][10] Prodelphinidin C2 (gallocatechin-(4α→8)-gallocatechin-(4α→8)-catechin) can be isolated in malt.[11]
The A-type proanthocyanidin epigallocatechin-(2β→7,4β→8)-epicatechin can be found in the leaves of Dioclea lasiophylla,[12]
See also
- Crofelemer, a complex mixture of procyanidins and prodelphinidins from the latex of the South American tree Croton lechleri (locally called Sangre de Grado or Sangre de Drago)
References
- Porter, 1992
- Rahima, Afidah A.; Roccab, Emmanuel; Steinmetzb, Jean; Kassima, M. Jain; Ibrahima, M. Sani; Osman, Hasnah (1 March 2008). "Antioxidant activities of mangrove Rhizophora apiculata bark extracts". Food Chemistry. 107 (1): 200–207. doi:10.1016/j.foodchem.2007.08.005.
- Prodelphinidin polymers: definition of structural units. Lai Yeap Foo and Lawrence J. Porter. J. Chem. Soc., Perkin Trans. 1, 1978, pp. 1186–1190, doi:10.1039/P19780001186
- Polymeric proanthocyanidins from grape skins. Jean-Marc Souquet, Véronique Cheynier, Franck Brossaud and Michel Moutounet, Phytochemistry, volume 43, Issue 2, September 1996, Pages 509–512, doi:10.1016/0031-9422(96)00301-9
- Flavan-3-ols, prodelphinidins and further polyphenols from Cistus salvifolius. Andreas Danne, Frank Petereit and Adolf Nahrstedt, Phytochemistry, Volume 37, Issue 2, 1994, Pages 533–538, doi:10.1016/0031-9422(94)85094-1
- Antioxidant properties of gallocatechin and prodelphinidins from pomegranate peel. Plumb G. W., de Pascual-Teresa S, Santos-Buelga C, Rivas-Gonzalo J. C and Williamson G, Redox Report, February 2002, Volume 7, Number 1, pages 41–46, doi:10.1179/135100002125000172
- Cheng HY, Lin CC, Lin TC (July 2002). "Antiviral properties of prodelphinidin B-2 3'-O-gallate from green tea leaf". Antivir. Chem. Chemother. 13 (4): 223–229. doi:10.1177/095632020201300403. PMID 12495210.
- Cheng HY, Lin TC, Ishimaru K, Yang CM, Wang KC, Lin CC (October 2003). "In vitro antiviral activity of prodelphinidin B-2 3,3'-di-O-gallate from Myrica rubra". Planta Med. 69 (10): 953–956. doi:10.1055/s-2003-45108. PMID 14648402.
- Delcour, Jan (1985). Structure elucidation of proanthocyanidins: Direct synthesis and isolation from Pilsener beer (PhD thesis). Katholieke Universiteit Leuven.
- Phenolic Compounds in Beer. Clarissa Gerhäuser and Hans Becker, Beer in Health and Disease Prevention, 2009 (article)
- Papagiannopoulos, Menelaos; Zimmermann, Benno; Mellenthin, Annett; Krappe, Martin; Maio, Giovanni; Galensa, Rudolf (7 June 2002). "Online coupling of pressurized liquid extraction, solid-phase extraction and high-performance liquid chromatography for automated analysis of proanthocyanidins in malt". Journal of Chromatography A. 958 (1–2): 9–16. doi:10.1016/S0021-9673(02)00364-3. PMID 12134835.
- Barreiros, André L. B. S.; David, Juceni P.; de Queiroz, Luciano P.; David, Jorge M. (December 2000). "A-type proanthocyanidin antioxidant from Dioclea lasiophylla". Phytochemistry. 55 (7): 805–808. doi:10.1016/S0031-9422(00)00297-1. PMID 11190400.