Proton-transfer-reaction mass spectrometry
Proton-transfer-reaction mass spectrometry (PTR-MS) is an analytical chemistry technique that uses gas phase hydronium reagent ions which are produced in an ion source.[1] PTR-MS is used for online monitoring of volatile organic compounds (VOCs) in ambient air and was developed in 1995 by scientists at the Institut für Ionenphysik at the Leopold-Franzens University in Innsbruck, Austria.[2] A PTR-MS instrument consists of an ion source that is directly connected to a drift tube (in contrast to SIFT-MS no mass filter is interconnected) and an analyzing system (quadrupole mass analyzer or time-of-flight mass spectrometer). Commercially available PTR-MS instruments have a response time of about 100 ms and reach a detection limit in the single digit pptv or even ppqv region. Established fields of application are environmental research, food and flavor science, biological research, medicine, security, cleanroom monitoring, etc.[1]
Theory
With H3O+ as the reagent ion the proton transfer process is (with R being the trace component)
-
(1)
Reaction (1) is only possible if energetically allowed, i.e. if the proton affinity of R is higher than the proton affinity of H2O (691 kJ/mol[3]). As most components of ambient air possess a lower proton affinity than H2O (e.g. N2, O2, Ar, CO2, etc.) the H3O+ ions only react with VOC trace components and the air itself acts as a buffer gas. Moreover, due to the low concentrations of trace components one can assume that the total number of H3O+ ions remains nearly unchanged, which leads to the equation[4]
-
(2)
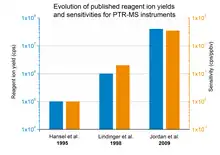
In equation (2) is the density of product ions, is the density of reagent ions in absence of reactant molecules in the buffer gas, k is the reaction rate constant and t is the average time the ions need to pass the reaction region. With a PTR-MS instrument the number of product and of reagent ions can be measured, the reaction rate constant can be found in literature for most substances[5] and the reaction time can be derived from the set instrument parameters. Therefore, the absolute concentration of trace constituents can be easily calculated without the need of calibration or gas standards. Furthermore, it gets obvious that the overall sensitivity of a PTR-MS instrument is dependent on the reagent ion yield. Fig. 1 gives an overview of several published (in peer-reviewed journals) reagent ion yields during the last decades and the corresponding sensitivities.
Technology

In commercial PTR-MS instruments water vapor is ionized in a hollow cathode discharge:
- .
After the discharge a short drift tube is used to form very pure (>99.5%[4]) H3O+ via ion-molecule reactions:
- .
Due to the high purity of the reagent ions a mass filter between the ion source and the reaction drift tube is not necessary and H3O+ can be injected directly. The absence of this mass filter in turn greatly reduces losses of reagent ions and leads eventually to an outstandingly low detection limit of the whole instrument. In the reaction drift tube a vacuum pump is continuously drawing through air containing the VOCs one wants to analyze. At the end of the drift tube the protonated molecules are mass analyzed (quadrupole mass analyzer or time-of-flight mass spectrometer) and detected.
As an alternative to H3O+ already in early PTR-MS related publications the use of NH4+ reagent ions has been suggested.[4] Ammonia has a proton affinity of 853.6 kJ/mol.[6] For compounds that have a higher proton affinity than ammonia proton transfer can take place similar to the process described above for hydronium:
- .
Additionally, for compounds with higher, but also for some with lower proton affinities than ammonia a clustering reaction can be observed
- *
where the cluster needs a third body to get collisionally stabilized. The main advantage of using NH4+ reagent ions is that fragmentation of analytes upon chemical ionization is strongly suppressed, leading to straightforward mass spectra even for complex mixtures. The reason why during the first 20 years after the invention of PTR-MS NH4+ reagent ions have only been used in a very limited number of studies is most probably because the NH4+ production required toxic and corrosive ammonia as a source gas. This led to problems with handling the instrument and its exhaust gas, as well as to increased wear of vacuum components. In 2017 a patent application was submitted where the inventors introduced a novel method of NH4+ production without the need of any form of ammonia.[7] In this method N2 and water vapor are introduced into the hollow cathode ion source and by adjusting electric fields and pressures NH4+ can be produced at the same or even higher purity levels than H3O+. It is expected that this invention, which eliminates the problems connected to the use of NH4+ so far, will lead to a widespread use of NH4+ reagent ions in the near future.[8]
Advantages
Advantages include low fragmentation – only a small amount of energy is transferred during the ionization process (compared to e.g. electron ionization), therefore fragmentation is suppressed and the obtained mass spectra are easily interpretable, no sample preparation is necessary – VOC containing air and liquids' headspaces can be analyzed directly, real-time measurements – with a typical response time of 100 ms VOCs can be monitored on-line, real-time quantification – absolute concentrations are obtained directly without previous calibration measurements, compact and robust setup – due to the simple design and the low number of parts needed for a PTR-MS instrument, it can be built in into space saving and even mobile housings, easy to operate – for the operation of a PTR-MS only electric power and a small amount of distilled water are needed. Unlike other techniques no gas cylinders are needed for buffer gas or calibration standards.
Disadvantages
One disadvantage is that not all molecules are detectable. Because only molecules with a proton affinity higher than water can be detected by PTR-MS, proton transfer from H3O+ is not suitable for all fields of application. Therefore, in 2009 first PTR-MS instruments were presented, which are capable of switching between H3O+ and O2+ (and NO+) as reagent ions.[9] This enhances the number of detectable substances to important compounds like ethylene, acetylene, most halocarbons, etc. Furthermore, particularly with NO+ it is possible to separate and independently quantify some isomers.[9] In 2012 a PTR-MS instrument was introduced which extends the selectable reagent ions to Kr+ and Xe+;[10] this should allow for the detection of nearly all possible substances (up to the ionization energy of krypton (14 eV[11])). Although the ionization method for these additional reagent ions is charge-exchange rather than proton-transfer ionization the instruments can still be considered as "classic" PTR-MS instruments, i.e. no mass filter between the ion source and the drift tube and only some minor modifications on the ion source and vacuum design.
The maximum measurable concentration is limited. Equation (2) is based on the assumption that the decrease of reagent ions is negligible, therefore the total concentration of VOCs in air must not exceed about 10 ppmv. Otherwise the instrument's response will not be linear anymore and the concentration calculation will be incorrect. This limitation can be overcome easily by diluting the sample with a well-defined amount of pure air.
Sensitivity enhancing measures
As it is the case for most analytical instruments, also in PTR-MS there has always been a quest for sensitivity improvement and for lowering the detection limit. However, until 2012 these improvements were limited to optimizations of the conventional setup, i.e. ion source, DC drift tube, transfer lens system, mass spectrometer (compare above). The reason for this conservative approach was that the addition of any RF ion focusing device negatively affects the well-defined PTR-MS ion chemistry, which makes quantification complicated and considerably limits comparability of measurement results obtained with different instruments. Only in 2016 a patent application providing a solution to this problem was submitted.[12]
Ion funnel
Ion funnels are RF devices which have been used for decades to focus ion currents into narrow beams. In PTR-MS they have been introduced in 2012 by Barber et al.[13] when they presented a PTR-MS setup with a PTR reaction region incorporating an ion funnel. Although the focusing properties of the ion funnel improved the sensitivity of the setup by a factor of >200 (compared to operating in DC only mode, i.e. with the ion funnel turned off) for some compounds, the sensitivities of other compounds were only improved by a factor of <10.[13] That is, because of the highly compound dependent instrumental response one of the main advantages of PTR-MS, namely that concentration values can be directly calculated, is lost and a calibration measurement is needed for each analyte of interest. Furthermore, with this approach unusual fragmentation of analytes has been observed[14] which complicates interpretation of measurement results and comparison between different types of instruments even more. A different concept has been introduced by the company IONICON Analytik GmbH.[15] (Innsbruck, AT) where the ion funnel is not predominantly part of the reaction region but mainly for focusing the ions into the transfer region to the TOF mass spectrometer.[16] In combination with the above-mentioned method of controlling the ion chemistry[12] this enables a considerable increase in sensitivity and thus also an improvement of the detection limit, while keeping the ion chemistry well-defined and thus avoiding problems with quantification and interpretation of the results.
Ion guide
Quadrupole, hexapole and other multipole ion guides can be used to transfer ions between different parts of an instrument with high efficiency. In PTR-MS they are particularly suitable for being installed in the differentially pumped interface between the reaction region and the mass spectrometer. In 2014 Sulzer et al.[17] published an article about a PTR-MS instrument which utilizes a quadrupole ion guide between the drift tube and the TOF mass spectrometer. They reported an increase in sensitivity by a factor of 25 compared to a similar instrument without an ion guide. Quadrupole ion guides are known to have high focusing power, but also rather narrow m/z transmission bands.[18] Hexapole ion guides on the other hand have focusing capabilities over a broader m/z band. Additionally, less energy is put into the transmitted ions, i.e. fragmentation and other adverse effects are less likely to occur. Consequently, some latest high-end PTR-MS instruments are equipped with hexapole ion guides for considerably improved performance[16] or even with a sequential arrangement of an ion funnel followed by a hexapole ion guide for even higher sensitivity and lower detection limit.[19]
Add-Ons
As a real-time trace gas analysis method based on mass spectrometry PTR-MS has two obvious limitations: Isomers cannot be easily separated (for some it is possible by switching the reagent ions[9] and/or by changing the reduced electric field strength in the drift tube) and the sample has to be in the gas phase. Countermeasures against these limitations have been developed in the form of add-ons, which can either be installed into the PTR-MS instrument or operated as external devices.
FastGC
Gas chromatography (GC) in combination with mass spectrometry (GC-MS) is capable of separating isomeric compounds. Although GC has been successfully coupled to PTR-MS in the past,[20] this approach annihilates the real-time capability of the PTR-MS technology, because a single GC analysis run typically takes between 30 min and 1 h. Thus, state-of-the-art GC add-ons for PTR-MS are based on fastGC technology. Materic et al.[21] utilized an early version of a commercially available fastGC addon in order to distinguish various monoterpene isomers. Within a fastGC run of about 70 s they were able to separate and identify: alpha-pinene, beta-pinene, camphene, myrcene, 3-carene and limonene in a standard mixture, Norway spruce, Scots pine and black pine samples, respectively. Particularly, if the operation mode of a PTR-MS instrument equipped with fastGC is continuously switched between fastGC and direct injection (dependent on the application, e.g. a loop sequence of one fastGC run followed by 10 min of direct injection measurement), real-time capability is preserved, while at the same time valuable information on substance identification and isomer separation is acquired.
Aerosol and particulate matter inlet
Researchers at the Leopold-Franzens University in Innsbruck invented a dedicated PTR-MS inlet system for the analysis of aerosols and particulate matter,[22] which they called "CHemical Analysis of aeRosol ON-line (CHARON)". After further development work in collaboration with a PTR-MS manufacturer, CHARON has become readily available as an add-on for PTR-MS instruments in 2017.[23] The add-on consists of a honeycomb activated charcoal denuder which adsorbs organic gases but transmits particles, an aerodynamic lens system that collimates sub-µm particles, and a thermo-desorber that evaporates non-refractory organic particulate matter at moderate temperatures of 100-160 °C and reduced pressures of a few mbar. So far, CHARON has predominantly being utilized within studies in the field of atmospheric chemistry, e.g. for airborne measurements of particulate organic matter[24] and bulk organic aerosol analysis.[25]
Inlet for liquids
A now well established setup for the controlled evaporation and subsequent analysis of liquids with PTR-MS has been published in 2013 by Fischer et al.[26] As the authors saw the main application of their setup in the calibration of PTR-MS instruments via aqueous standards, they named it "Liquid Calibration Unit (LCU)". The LCU sprays a liquid standard into a gas stream at well-defined flow rates via a purpose-built nebulizer (optimized for reduced probability of clogging and high tolerance to salts in the liquid). The resulting micro-droplets are injected into a heated (> 100 °C) evaporation chamber. This concept offers two main advantages: (i) the evaporation of compounds is enhanced by the enlarged surface area of the droplets and (ii) compounds which are dissociated in water, such as acids (or bases), experience a shift in pH value when the water evaporates from a droplet. This in turn reduces dissociation and supports total evaporation of the compound.[26] The resulting continuous gas flow containing the analytes can be directly introduced into a PTR-MS instrument for analysis.
Applications
The most common applications for the PTR-MS technique are environmental research,[27][28][29] waste incineration, food science,[30] biological research,[31] process monitoring, indoor air quality,[32][33][34] medicine and biotechnology[35][36][37][38] and Homeland security.[39][40] Trace gas analysis is another common application. Some other techniques are Secondary electrospray ionization (SESI), Electrospray ionization (ESI), and Selected-ion flow-tube mass spectrometry (SIFT).
Food science

Fig. 2 shows a typical PTR-MS measurement performed in food and flavor research. The test person swallows a sip of a vanillin flavored drink and breathes via his nose into a heated inlet device coupled to a PTR-MS instrument. Due to the high time resolution and sensitivity of the instrument used here, the development of vanillin in the person's breath can be monitored in real-time (please note that isoprene is shown in this figure because it is a product of human metabolism and therefore acts as an indicator for the breath cycles). The data can be used for food design, i.e. for adjusting the intensity and duration of vanillin flavor tasted by the consumer.

Another example for the application of PTR-MS in food science was published in 2008 by C. Lindinger et al.[42] in Analytical Chemistry. This publication found great response even in non-scientific media.[43][44] Lindinger et al. developed a method to convert "dry" data from a PTR-MS instrument that measured headspace air from different coffee samples into expressions of flavor (e.g. "woody", "winey", "flowery", etc.) and showed that the obtained flavor profiles matched nicely to the ones created by a panel of European coffee tasting experts.
Air quality analysis
In Fig. 3 a mass spectrum of air inside a laboratory (obtained with a time-of-flight (TOF) based PTR-MS instrument), is shown. The peaks on m/z 19, 37 and 55 (and their isotopes) represent the reagent ions (H3O+) and their clusters. On m/z 30 and 32 NO+ and O2+, which are both impurities originating from the ion source, appear. All other peaks correspond to compounds present in typical laboratory air (e.g. high intensity of protonated acetone on m/z 59). If one takes into account that virtually all peaks visible in Fig. 3 are in fact double, triple or multiple peaks (isobaric compounds) it becomes obvious that for PTR-MS instruments selectivity is at least as important as sensitivity, especially when complex samples / compositions are analyzed. One methods for improving the selectivity is high mass resolution. When the PTR source is coupled to a high resolution mass spectrometer isobaric compounds can be distinguished and substances can be identified via their exact mass.[45] Some PTR-MS instruments are despite of the lack of a mass filter between the ion source and the drift tube capable of switching the reagent ions (e.g. to NO+ or O2+). With the additional information obtained by using different reagent ions a much higher level of selectivity can be reached, e.g. some isomeric molecules can be distinguished.[9]
See also
References
- Andrew M. Ellis; Christopher A. Mayhew (17 December 2013). Proton Transfer Reaction Mass Spectrometry: Principles and Applications. Wiley. pp. 15–. ISBN 978-1-118-68412-2.
- A. Hansel, A. Jordan, R. Holzinger, P. Prazeller W. Vogel, W. Lindinger, Proton transfer reaction mass spectrometry: on-line trace gas analysis at ppb level, Int. J. of Mass Spectrom. and Ion Proc., 149/150, 609-619 (1995).
- R.S. Blake, P.S. Monks, A.M. Ellis, Proton-Transfer Reaction Mass Spectrometry, Chem. Rev., 109, 861-896 (2009)
- Lindinger, W.; Hansel, A.; Jordan, A. (1998). "On-line monitoring of volatile organic compounds at pptv levels by means of Proton-Transfer-Reaction Mass-Spectrometry (PTR-MS): Medical applications, food control and environmental research, Review paper". Int. J. Mass Spectrom. Ion Process. 173 (3): 191–241. Bibcode:1998IJMSI.173..191L. doi:10.1016/s0168-1176(97)00281-4.
- Y. Ikezoe, S. Matsuoka and A. Viggiano, Gas Phase Ion-Molecule Reaction Rate Constants through 1986, Maruzen Company Ltd., Tokyo, (1987).
- "Ammonia". webbook.nist.gov.
- 20181220 WO application WO2018EP86332 20181220, Hartungen, Eugen, "Method for Producing Gaseous Ammonium for Ion-Molecule-Reaction Mass Spectrometry", published 2019-06-27, assigned to IONICON Analytik GmbH.
- Müller, Markus; Piel, Felix; Gutmann, Rene; Sulzer, Philipp; Hartungen, Eugen; Wisthaler, Eugen (2019). "A novel method for producing NH4+ reagent ions in the hollow cathode glow discharge ion source of PTR-MS instruments". Int. J. Mass Spectrom. 447: 116254. doi:10.1016/j.ijms.2019.116254.
- Jordan, A.; Haidacher, S.; Hanel, G.; Hartungen, E.; Herbig, J.; Märk, L.; Schottkowsky, R.; Seehauser, H.; Sulzer, P.; Märk, T.D. (2009). "An online ultra-high sensitivity proton-transfer-reaction mass-spectrometer combined with switchable reagent ion capability (PTR+SRI-MS)". International Journal of Mass Spectrometry. 286 (1): 32–38. Bibcode:2009IJMSp.286...32J. doi:10.1016/j.ijms.2009.06.006.
- Sulzer, P.; Edtbauer, A.; Hartungen, E.; Jürschik, S.; Jordan, A.; Hanel, G.; Feil, S.; Jaksch, S.; Märk, L.; Märk, T. D. (2012). "From conventional Proton-Transfer-Reaction Mass Spectrometry (PTR-MS) to universal trace gas analysis". International Journal of Mass Spectrometry. 321–322: 66–70. Bibcode:2012IJMSp.321...66S. doi:10.1016/j.ijms.2012.05.003.
- "Krypton". webbook.nist.gov.
- US patent 10074531, Sulzer, Philipp; Jürschik, Simone & Herbig, Jens et al., issued 2018-09-11, assigned to IONICON Analytik Gesellschaft m.b.H.
- Barber, Shane; Blake, Robert S.; White, Iain R.; Monks, Paul S.; Reich, Fraser; Mullock, Steve; Ellis, Andrew M. (2012). "Increased Sensitivity in Proton Transfer Reaction Mass Spectrometry by Incorporation of a Radio Frequency Ion Funnel". Anal. Chem. 84 (12): 5387–5391. doi:10.1021/ac300894t. hdl:2381/13487. PMID 22612412.
- Gonzalez-Mendez, Ramon; Watts, Peter; Olivenza-Leon, David; Reich, D. Fraser; Mullock, Stephen D.; Corlett, Clive A.; Cairns, Stuart; Hickey, Peter; Brookes, Matthew; Mayhew, Chris A. (2016). "Enhancement of Compound Selectivity Using a Radio Frequency Ion-Funnel Proton Transfer Reaction Mass Spectrometer: Improved Specificity for Explosive Compounds". Anal. Chem. 88 (21): 10624–10630. doi:10.1021/acs.analchem.6b02982. PMID 27715015.
- "IONICON Website". www.ionicon.com.
- Yuan, Bin; Koss, Abigail R.; Warneke, Carsten; Coggon, Matthew; Sekimoto, Kanako; de Gouw, Joost A. (2017). "Proton-Transfer-Reaction Mass Spectrometry: Applications in Atmospheric Sciences". Chem. Rev. 117 (21): 13187–13229. doi:10.1021/acs.chemrev.7b00325. PMID 28976748.
- Sulzer, Philipp; Hartungen, Eugen; Hanel, Gernot; Feil, Stefan; Winkler, Klaus; Mutschlechner, Paul; Haidacher, Stefan; Schottkowsky, Ralf; Gunsch, Daniel; Seehauser, Hans; Striednig, Marcus; Jürschik, Simone; Breiev, Kostiantyn; Lanza, Matteo; Herbig, Jens; Märk, Lukas; Märk, Tilmann D.; Jordan, Alfons (2014). "A Proton Transfer Reaction-Quadrupole interface Time-Of-Flight Mass Spectrometer (PTR-QiTOF): High speed due to extreme sensitivity". Int. J. Mass Spectrom. 368: 1–5. doi:10.1016/j.ijms.2014.05.004.
- Gerlich, Dieter (1992-01-01). "Inhomogeneous RF Fields: A Versatile Tool for the Study of Processes with Slow Ions". In Ng, Cheuk-Yiu; Baer, Michael; Prigogine, Ilya; Rice, Stuart A. (eds.). Advances in Chemical Physics. Vol. 82. pp. 1–176. doi:10.1002/9780470141397.ch1. ISBN 9780470141397.
- Piel, Felix; Winkler, Klaus; Gutmann, Rene; Haidacher, Stefan; Herbig, Jens; Mayramhof, Gregor; Jürschik, Simone; Jordan, Alfons; Märk, Lukas; Sulzer, Philipp (2018-08-01). "A sophisticated setup for rapid, sensitive and selective food and flavor analysis". In Siegmund, Barbara; Leitner, Erich (eds.). Flavour Science. 15th Weurman Flavour Research Symposium. Verlag der Technischen Universität Graz. pp. 433–438. doi:10.3217/978-3-85125-593-5. ISBN 978-3-85125-594-2.
- Lindinger, Christian; Pollien, Philippe; Ali, Santo; Yeretzian, Chahan; Blank, Imre; Märk, Tilmann (2005). "Unambiguous Identification of Volatile Organic Compounds by Proton-Transfer Reaction Mass Spectrometry Coupled with GC/MS". Anal. Chem. 77 (13): 4117–4124. doi:10.1021/ac0501240. PMID 15987117.
- Materic, Dusan; Lanza, Matteo; Sulzer, Philipp; Herbig, Jens; Bruhn, Dan; Turner, Claire; Mason, Nigel; Gauci, Vincent (2015). "Monoterpene separation by coupling proton transfer reaction time-of-flight mass spectrometry with fastGC" (PDF). Anal Bioanal Chem. 407 (25): 7757–7763. doi:10.1007/s00216-015-8942-5. PMID 26253230. S2CID 25553133.
- Eichler, P.; Müller, M.; D'Anna, B.; Wisthaler, A. (2015). "A novel inlet system for online chemical analysis of semi-volatile submicron particulate matter". Atmos. Meas. Tech. 8 (3): 1353–1360. doi:10.5194/amt-8-1353-2015.
- Märk, Lukas (2017-10-25). "CHARON Real-Time Aerosol Inlet System for PTR-TOFMS". IONICON blog. Retrieved 2020-03-18.
CHARON is now available for selected PTR-TOFMS instruments, exclusively from IONICON.
- Piel, Felix; Müller, Markus; Mikoviny, Thomas; Pusede, Sally E.; Wisthaler, Armin (2019). "Airborne measurements of particulate organic matter by proton-transfer-reaction mass spectrometry (PTR-MS): a pilot study". Atmos. Meas. Tech. 12 (11): 5947–5958. doi:10.5194/amt-12-5947-2019.
- Leglise, Joris; Müller, Markus; Piel, Felix; Otto, Tobias; Wisthaler, Armin (2019). "Bulk Organic Aerosol Analysis by Proton-Transfer-Reaction Mass Spectrometry: An Improved Methodology for the Determination of Total Organic Mass, O:C and H:C Elemental Ratios, and the Average Molecular Formula" (PDF). Anal. Chem. 91 (20): 12619–12624. doi:10.1021/acs.analchem.9b02949. PMID 31525909. S2CID 202675060.
- Fischer, Lukas; Klinger, Andreas; Herbig, Jens; Winkler, Klaus; Gutmann, Rene; Hansel, Armin (2013). "The LCU: Versatile Trace Gas Calibration" (PDF). In Hansel, Armin; Dunkl, Jürgen (eds.). Conference Series. 6th International Conference on Proton Transfer Reaction Mass Spectrometry and its Applications. innsbruck university press. pp. 192–195. ISBN 978-3-902811-91-2.
- de Gouw, J.; Warneke, C.; Karl, T.; Eerdekens, G.; van der Veen, C.; Fall, R. (2007). "Measurement of Volatile Organic Compounds in the Earth's Atmosphere using Proton-Transfer-Reaction Mass Spectrometry". Mass Spectrometry Reviews. 26 (2): 223–257. Bibcode:2007MSRv...26..223D. doi:10.1002/mas.20119. PMID 17154155.
- Müller, M.; Graus, M.; Ruuskanen, T. M.; Schnitzhofer, R.; Bamberger, I.; Kaser, L.; Titzmann, T.; Hörtnagl, L.; Wohlfahrt, G.; Karl, T.; Hansel, A. (2010). "First eddy covariance flux measurements by PTR-TOF". Atmos. Meas. Tech. 3 (2): 387–395. doi:10.5194/amt-3-387-2010. PMC 3898015. PMID 24465280.
- R. Beale, P. S. Liss, J. L. Dixon, P. D. Nightingale: Quantification of oxygenated volatile organic compounds in seawater by membrane inlet-proton transfer reaction/mass spectrometry. Anal. Chim. Acta (2011).
- F. Biasioli, C. Yeretzian, F. Gasperi, T. D. Märk: PTR-MS monitoring of VOCs and BVOCs in food science and technology, Trends in Analytical Chemistry, 30/7, (2011).
- Simpraga, M.; Verbeeck, H.; Demarcke, M.; Joó, É.; Pokorska, O.; Amelynck, C.; Schoon, N.; Dewulf, J.; Langenhove, H. Van; Heinesch, B.; Aubinet, M.; Laffineur, Q.; Müller, J.-F.; Steppe, K. (2011). "Clear link between drought stress, photosynthesis and biogenic volatile organic compounds in Fagus sylvatica L". Atmospheric Environment. 45 (30): 5254–5259. Bibcode:2011AtmEn..45.5254S. doi:10.1016/j.atmosenv.2011.06.075.
- Wisthaler, A.; Strom-Tejsen, P.; Fang, L.; Arnaud, T. J.; Hansel, A.; Märk, T. D.; Wyon, D. P. (2007). "PTR-MS Assessment of Photocatalytic and Sorption-Based Purification of Recirculated Cabin Air during Simulated 7-h Flights with High Passenger Density". Environ. Sci. Technol. 1 (1): 229–234. Bibcode:2007EnST...41..229W. doi:10.1021/es060424e. PMID 17265952.
- Kolarik, B.; Wargocki, P.; Skorek-Osikowska, A.; Wisthaler, A. (2010). "The effect of a photocatalytic air purifier on indoor air quality quantified using different measuring methods". Building and Environment. 45 (6): 1434–1440. doi:10.1016/j.buildenv.2009.12.006.
- Han, K.H.; Zhang, J.S.; Knudsen, H.N.; Wargocki, P.; Chen, H.; Varshney, P.K.; Guo, B. (2011). "Development of a novel methodology for indoor emission source identification". Atmospheric Environment. 45 (18): 3034–3045. Bibcode:2011AtmEn..45.3034H. doi:10.1016/j.atmosenv.2011.03.021.
- Herbig, J.; Müller, M.; Schallhart, S.; Titzmann, T.; Graus, M.; Hansel, A. (2009). "On-line breath analysis with PTR-TOF". Journal of Breath Research. 3 (2): 027004. Bibcode:2009JBR.....3b7004H. doi:10.1088/1752-7155/3/2/027004. PMID 21383459. S2CID 2583809.
- Brunner, C.; Szymczak, W.; Höllriegl, V.; Mörtl, S.; Oelmez, H.; Bergner, A.; Huber, R. M.; Hoeschen, C.; Oeh, U. (2010). "Discrimination of cancerous and non-cancerous cell lines by headspace-analysis with PTR-MS". Anal. Bioanal. Chem. 397 (6): 2315–2324. doi:10.1007/s00216-010-3838-x. PMID 20502883. S2CID 31066400.
- Blake, R. S.; Monks, P. S.; Ellis, A. M. (2009). "Proton-Transfer Reaction Mass Spectrometry". Chem. Rev. 109 (3): 861–896. doi:10.1021/cr800364q. PMID 19215144.
- Jens Herbig and Anton Amann " Proton Transfer Reaction-Mass Spectrometry Applications in Medical Research" Journal of Breath Research Volume 3, Number 2, June 2009.
- Jürschik, S.; Sulzer, P.; Petersson, F.; Mayhew, C. A.; Jordan, A.; Agarwal, B.; Haidacher, S.; Seehauser, H.; Becker, K.; Märk, T. D. (2010). "Proton transfer reaction mass spectrometry for the sensitive and rapid real-time detection of solid high explosives in air and water". Anal Bioanal Chem. 398 (7–8): 2813–2820. doi:10.1007/s00216-010-4114-9. PMID 20803004. S2CID 26279076.
- Petersson, F.; Sulzer, P.; Mayhew, C.A.; Watts, P.; Jordan, A.; Märk, L.; Märk, T.D. (2009). "Real-time trace detection and identification of chemical warfare agent simulants using recent advances in proton transfer reaction time-of-flight mass spectrometry, Rapid Commun". Mass Spectrom. 23 (23): 3875–3880. doi:10.1002/rcm.4334. PMID 19902419.
- Hartungen, Eugen; Jürschik, Simone; Jordan, Alfons; Edtbauer, Achim; Feil, Stefan; Hanel, Gernot; Seehauser, Hans; Haidacher, Stefan; Schottkowsky, Ralf; Märk, Lukas; Jaksch, Stefan; Agarwal, Bishu; Becker, Kurt; Mayhew, Chris A.; Sulzer, Philipp; Märk, Tilmann D. (2013). "Proton transfer reaction-mass spectrometry: fundamentals, recent advances and applications". Eur. Phys. J. Appl. Phys. 61 (2): 24303. doi:10.1051/epjap/2012120401.
- C. Lindinger, D. Labbe, P. Pollien, A. Rytz, M. A. Juillerat, C. Yeretzian, I. Blank, 2008 When Machine Tastes Coffee: Instrumental Approach To Predict the Sensory Profile of Espresso Coffee, Anal. Chem., 80/5, 1574-1581.
- "MSN - Outlook, Office, Skype, Bing, Breaking News, and Latest Videos". NBC News. 10 March 2008.
- Fountain, Henry (2008-02-19). "Scientists Finding Ways to Perfect a Cup of Joe, Without the Attitude". The New York Times.
- A. Jordan, S. Haidacher, G. Hanel, E. Hartungen, L. Märk, H. Seehauser, R. Schottkowsky, P. Sulzer, T.D. Märk: A high resolution and high sensitivity time-of-flight proton-transfer-reaction mass spectrometer (PTR-TOF-MS), International Journal of Mass Spectrometry, 286, 122–128, (2009).