Pulegone
 | |
| Names | |
|---|---|
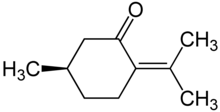
| Preferred IUPAC name
(5R)-5-Methyl-2-(propan-2-ylidene)cyclohexan-1-one | |
| Other names
p-Menth-4(8)-en-3-one; δ-4(8)-p-Menthen-3-one; (R)-2-Isopropylidene-5-methylcyclohexanone; (R)-p-Menth-4(8)-en-3-one; (R)-(+)-Pulegone | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.001.767 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H16O | |
| Molar mass | 152.237 g·mol−1 |
| Appearance | Colorless oil |
| Density | 0.9346 g/cm3 |
| Boiling point | 224 °C (435 °F; 497 K) |
| Insoluble | |
| Solubility in Ethanol Ether Chloroform |
Miscible |
| Hazards | |
| Safety data sheet (SDS) | MSDS[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Pulegone is a naturally occurring organic compound obtained from the essential oils of a variety of plants such as Nepeta cataria (catnip), Mentha piperita, and pennyroyal.[3][4] It is classified as a monoterpene.
Pulegone is a clear colorless oily liquid and has a pleasant odor similar to pennyroyal, peppermint and camphor. It is used in flavoring agents, in perfumery, and in aromatherapy.
Toxicology
It was reported that the chemical is toxic to rats if a large quantity is consumed.[5][6]
Pulegone is also an insecticide − the most powerful of three insecticides naturally occurring in many mint species.[7]
As of October 2018, the FDA withdrew authorization for the use of pulegone as a synthetic flavoring substance for use in food, but that naturally-occurring pulegone can continue to be used.[8]
Sources
See also
References
- Merck Index, 11th Edition, 7955.
- Universiti Malaysia Pahang. "Safety data sheet" (PDF). Retrieved 8 June 2009.
- Grundschober, F. (1979). "Literature review of pulegone". Perfum. Flavorist. 4: 15–17.
- Sullivan, J.B., Rumack, B.H., Thomas, H., Peterson, R.G. & Brysch, P. (1979). "Pennyroyal oil poisoning and hepatotoxicity". J. Am. Med. Assoc. 242 (26): 2873–2874. doi:10.1001/jama.1979.03300260043027. PMID 513258.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Thorup, I.; Würtzen, G; Carstensen, J; Olsen, P; et al. (1983). "Short term toxicity study in rats dosed with pulegone and menthol". Toxicology Letters. 19 (3): 207–210. doi:10.1016/0378-4274(83)90120-0. PMID 6658833.
- Asekun, O.T.; Grierson, D; Afolayan, A; et al. (2006). "Effects of drying methods on the quality and quantity of the essential oil of Mentha longifolia L. subsp. Capensis". Food Chemistry. 101 (3): 995–998. doi:10.1016/j.foodchem.2006.02.052.
- Franzios, G; Mirotsou M; Hatziapostolou E; Kral J; Scouras ZG; Mavragani-Tsipidou P (16 July 1997). "Insecticidal and genotoxic activities of mint essential oils". Journal of Agricultural and Food Chemistry. 45 (7): 2690–2694. doi:10.1021/jf960685f. Archived from the original on 23 December 2012. Retrieved 19 October 2012.
- 83 FR 50490
- Božović M, Pirolli A, Ragno R (May 2015). "Mentha suaveolens Ehrh. (Lamiaceae) Essential Oil and Its Main Constituent Piperitenone Oxide: Biological Activities and Chemistry". Molecules (Basel, Switzerland). 20 (5): 8605–33. doi:10.3390/molecules20058605. PMC 6272761. PMID 25985361.
- Gordon, W. Perry; Valerie Howland; et al. (1982). "Hepatotoxicity and pulmonary toxicity of pennyroyal oil and its constituent terpenes in the mouse". Toxicology and Applied Pharmacology. 65 (3): 413–424. doi:10.1016/0041-008X(82)90387-8. PMID 7157374.
- Farley, Derek R.; Valerie Howland (2006). "The natural variation of the pulegone content in various oils of peppermint". Journal of the Science of Food and Agriculture. 31 (11): 1143–1151. doi:10.1002/jsfa.2740311104.