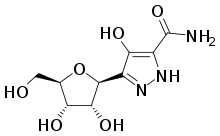
Pyrazofurin
Pyrazofurin (pyrazomycin) is a natural product found in Streptomyces candidus, which is a nucleoside analogue related to ribavirin. It has antibiotic, antiviral and anti-cancer properties but was not successful in human clinical trials due to severe side effects. Nevertheless, it continues to be the subject of ongoing research as a potential drug of last resort, or a template for improved synthetic derivatives.[1][2][3][4][5][6][7][8]
 | |
| Clinical data | |
|---|---|
| Trade names | Pyrazofurin |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C9H13N3O6 |
| Molar mass | 259.22 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
See also
References
- Canonico PG, Jahrling PB, Pannier WL (December 1982). "Antiviral efficacy of pyrazofurin against selected RNA viruses". Antiviral Research. 2 (6): 331–7. doi:10.1016/0166-3542(82)90002-x. PMID 6299188.
- Buchanan JG (1983). "The C-nucleoside antibiotics". Fortschritte der Chemie Organischer Naturstoffe. Fortschritte der Chemie organischer Naturstoffe / Progress in the Chemistry of Organic Natural Products. 44: 243–99. doi:10.1007/978-3-7091-8714-2_4. ISBN 978-3-7091-8716-6. PMID 6360831.
- Hacksell U, Daves GD (1985). "The chemistry and biochemistry of C-nucleosides and C-arylglycosides". Progress in Medicinal Chemistry. 22: 1–65. doi:10.1016/s0079-6468(08)70228-5. PMID 3915364.
- De Clercq E (July 2009). "Another ten stories in antiviral drug discovery (part C): "Old" and "new" antivirals, strategies, and perspectives". Medicinal Research Reviews. 29 (4): 611–45. doi:10.1002/med.20153. PMID 19260077. S2CID 140127449.
- De Clercq E (2015). "Curious (Old and New) Antiviral Nucleoside Analogues with Intriguing Therapeutic Potential". Current Medicinal Chemistry. 22 (34): 3866–80. doi:10.2174/0929867322666150625094705. PMID 26112146.
- De Clercq E (March 2016). "C-Nucleosides To Be Revisited". Journal of Medicinal Chemistry. 59 (6): 2301–11. doi:10.1021/acs.jmedchem.5b01157. PMID 26513594.
- De Clercq E (November 2019). "New Nucleoside Analogues for the Treatment of Hemorrhagic Fever Virus Infections". Chemistry: An Asian Journal. 14 (22): 3962–3968. doi:10.1002/asia.201900841. PMC 7159701. PMID 31389664.
- Ren D, Wang SA, Ko Y, Geng Y, Ogasawara Y, Liu HW (November 2019). "Identification of the C-Glycoside Synthases during Biosynthesis of the Pyrazole-C-Nucleosides Formycin and Pyrazofurin". Angewandte Chemie. 58 (46): 16512–16516. doi:10.1002/anie.201910356. PMC 6911263. PMID 31518483.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.