QSER1
Glutamine Serine Rich Protein 1 or QSER1 is a protein encoded by the QSER1 gene.[5]
| QSER1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | QSER1, glutamine and serine rich 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | MGI: 2138986 HomoloGene: 11710 GeneCards: QSER1 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
QSER1 protein is a DNA methylation regulator.[6] QSER1 has one alias, FLJ21924.[5]
Gene
Location
The QSER1 gene is found on the short arm of chromosome 11 (11p13), beginning at 32,914,792 bp and ending at 33,001,816 bp. It is 87,024 bp in length. It is located between the genes DEPDC7 and PRRG4 and is 500,000 bp downstream from the Wilms Tumor 1 gene (WT1), which is implicated in multiple pathologies.[5][7]
Orthologs
QSER1 is highly conserved in most species of the clade Chordata. Orthologs have been found in primates, birds, reptiles, amphibians, and fish as far back as the coelacanth, which diverged 414.9 million years ago.[5][7]
Paralogs
QSER1 has one paralog in humans, Proline-rich 12, or PRR12. PRR12 is found on chromosome 9 at 9q13.33, which does not have known function. PRR12 is found in most chordate species as far back as the coelacanth.[8] The duplication event likely occurred sometime in the chordate lineage near the divergence of the coelacanth. Both PRR12 and QSER1 contain the conserved DUF4211 domain near the 3’ ends of the genes.[5][8]
mRNA
Promoter and transcription factors
The promoter region for QSER1 is 683 bp in length and is found on chromosome 11 between 32,914,224 bp and 32,914,906. There is some overlap between the promoter region and the 5’ UTR of QSER1. Predicted transcription factors with conservation include (but are not limited to) EGR1, p53, E2F3, E2F4, PLAG1, NeuroD2, Myf5, IKZF1, SMAD3, KRAB, MZF1, and c-Myb.[9]
Normal expression
Expression of QSER1 is seen at levels lower than 50% in many tissues. However, notable expression is seen in skeletal muscle, the appendix, trigeminal ganglia, cerebellum peduncles, pons, spinal cord, ciliary ganglion, globus pallidus, subthalamic nucleus, dorsal root ganglion, fetal liver, adrenal gland, ovary, uterus corpus, cardiac myocytes, the atrioventricular node, skin, pituitary gland, tongue, early erythroid progenitors, and tonsil.[10][11]
Differential expression
A notable decrease in QSER1 expression has been noted in renal mesangial cells in response to treatment with 25 mM glucose. This condition was studied as differential expression of genes involved in cell cycle regulation had been noted in these cells in response to high glucose levels seen with diabetes mellitus.[12][13] A different study noted overexpression of QSER1 in pathological cardiomyopathy. This condition is associated with altered expression of genes involved in immune responses, signaling, cell growth, and proliferation as well as infiltration of B lymphocytes.[14][15]
Differential expression of QSER1 is seen in multiple cancer conditions. Overexpression of QSER1 was noted in Burkitt’s Lymphoma.[10] QSER1 expression also increases with increasing Gleason score (more advanced stages) of prostate cancer.[16] In a study on breast cancer response to paclitaxel and fluorouracil‐doxorubicin‐cyclophosphamide chemotherapy, it was noted that breast cancer lines with higher levels of QSER1 were more likely to respond to treatment than those with underexpression of QSER1.[17] Greater expression of QSER1 was also noted in mammary epithelial cells of immortalized cell lines than in mammary epithelial cells from cell lines with finite lifespan.[18]
Protein
General properties
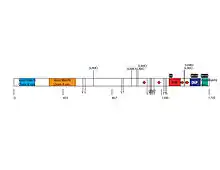
QSER1 protein is 1735 amino acids in length.[21] The composition of the peptide is significantly high in serine and glutamine: 14.7% serine residues and 8.9% glutamine.[22]
Conservation
QSER1 protein is highly conserved in chordate species. The table below shows information on the protein orthologs.
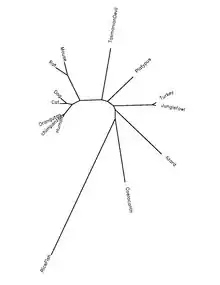
| Genus and species name | Common name | Protein accession number[23] | Sequence Identity to human protein[23] |
|---|---|---|---|
| Homo sapiens | Humans | NP_001070254.1 | |
| Pan troglodytes | Chimpanzee | XP_508354.3 | 99% |
| Macaca mulatta | Rhesus macaque | NP_001244647.1 | 98% |
| Callithris jacchus | Marmoset | XP_002755192.1 | 96% |
| Ailuropoda melanoleuca | Giant panda | XP_002917539.1 | 90% |
| Loxodonta africana | Elephant | XP_003412344.1 | 88% |
| Mus musculus | Mouse | NP_001116799.1 | 81% |
| Monodelphis domestica | Opossum | XP_001368629.1 | 71% |
| Ornithorhynchus anatinus | Platypus | XP_001506659.2 | 75% |
| Taeniopygia guttata | Zebra finch | XP_002195876.1 | 69% |
| Gallus gallus | Chicken | NP_001186343.1 | 69% |
| Anolis carolinensis | Carolina anole (lizard) | XP_003214747.1 | 62% |
| Takifugu rubripes | Japanese pufferfish | XP_003977915.1 | 48% |
| Latimeria chalumnae | Coelacanth | N/A | 62% |
Domains and motifs

QSER1 protein contains two high conserved domains found not only in QSER1 but also in other protein products. These include the PHA02939 domain from amino acid 1380-1440 and the DUF4211 domain from amino acid 1522-1642.[24][25] Nuclear localization was predicted by pSORT. This property was conserved from the human QSER1 to the coelacanth QSER1. Multiple conserved nuclear localization signals were also predicted within the QSER1 protein by pSORT.[26]
Structure
Predictions of the QSER1 protein structure indicate that the protein contains many alpha helices.[27][28][29] NCBI cBLAST predicted structural similarity between the QSER1 protein and the Schizosaccharomyces pombe (fission yeast) RNA Polymerase II A chain. The two regions of similarity occur between amino acids 56-194 and 322-546.[28] This first region (56-194) is a regulatory region in both the human and yeast RNA Polymerase II containing multiple repeats of the sequence YSPTSPSYS. Phosphorylation of serine residues in this region regulates progression through the steps of gene transcription.[30] A 3D structure was provided for this region. The structurally similar region is on the exterior of the protein molecule and forms part of the DNA binding cleft.
Further structural similarity to a viral RNA Polymerase binding protein was predicted by Phyre2.[29] This structure is found at the very end of the protein between amino acids 1671-1735. The structure has a long region of alpha helices that were also predicted by SDSC Biology Workbench PELE. An image of the structurally similar region and sequence alignment is shown on the right. Regions before the identified structurally similar domain show two other alpha helices predicted with high confidence.[29]
Phosphorylation

There are 12 confirmed phosphorylation sites on the QSER1 protein. Eight are phosphoserines, one phosphotyrosine, and three phosphothreonines. Three of these sites have been shown to be phosphorylated by ATM and ATR in response in DNA damage.[31] 123 other possible phosphorylation sites have been predicted using the ExPASy NetPhos tool.[32]
SUMOylation
Interaction of QSER1 protein with SUMO has been noted in multiple proteome-wide studies.[33][34] Predicted SUMOylation sites have been found in QSER1 protein. Highly conserved SUMOylation sites occur with the sequence MKMD at amino acid 794, VKIE at 1057, VKTG at 1145, LKSG at 1157, VKQP at 1487, and VKAE at 1492 .[35]

ATM/ATR
Phosphorylation of QSER1 at three serine residues, S1228, S1231, and S1239, by ATM and ATR in response to DNA damage was found in a proteome-wide study.[31]
SUMO
Interaction of QSER1 with SUMO has been confirmed in multiple studies.[33][34] The role of SUMOylation in QSER1 function is unclear. However, there may be a connection between QSER1 and SUMO in response to endoplasmic reticulum stress (often caused by accumulation of misfolded proteins). In a study on ER stress, QSER1 was tagged as an ER stress response gene with altered expression.[36] Further, in a study on SUMOylation in response to accumulated misfolded proteins and ER stress found QSER1 to be a SUMO interactant in this situation.[33] Any connection between these two activities is unstudied and unconfirmed.
RNA polymerase II
Direct interaction of QSER1 with RNA polymerase II was found in a study performed by Moller, et al. Interaction was shown to occur with the DNA-directed RNA polymerase II subunit, RPB1, of RNA polymerase II during both mitosis and interphase. Colocalization/interaction of QSER1 was shown to the regulatory region of RPB1 with 52 heptapeptide (YSPTSPSYS) repeats.[30]
NANOG and TET1
Interaction between homeobox protein NANOG and Tet methylcytosine dioxygenase 1 (TET1) has been shown to be important in establishing pluripotency during the generation of induced pluripotent stem cells. QSER1 protein was shown to interact with both NANOG and TET1.[37]
Pathology
Altered expression of QSER1 is noted in pathological cardiomyopathy, Burkitt's Lymphoma, prostate cancer, and some breast cancers mentioned above.[9][10][14][16] NCBI AceView lists multiple mutations associated with other pathologies including an eight base pair and 13 base pair deletion in QSER1 associated with leiomyosarcoma of the uterus, and 57 base pair difference in a neuroblastoma. Also listed are multiple splice variants with truncated 5' and/or 3' ends often noted in cancerous conditions.[40] Further, according to the NCBI OMIM database, multiple pathologies are associated with alterations in the 11p13 region and therefore may implicate QSER1.[41] These include Exudative Vitreoretinopathy 3,[42] Familial Candidiasis 3,[43] Centralopathic Epilepsy,[44] and Autosomal Recessive Deafness 51.[45] QSER1 was also noted as a susceptibility gene for Parkinson's disease.[36]
References
- GRCh38: Ensembl release 89: ENSG00000060749 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000074994 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "NCBI QSER1 Gene".
- Dixon, Gary; Pan, Heng; Yang, Dapeng; Rosen, Bess P.; Jashari, Therande; Verma, Nipun; Pulecio, Julian; Caspi, Inbal; Lee, Kihyun; Stransky, Stephanie; Glezer, Abigail; Liu, Chang; Rivas, Marco; Kumar, Ritu; Lan, Yahui; Torregroza, Ingrid; He, Chuan; Sidoli, Simone; Evans, Todd; Elemento, Olivier; Huangfu, Danwei (2021). "QSER1 protects DNA methylation valleys from de novo methylation". Science. 372 (6538): eabd0875. doi:10.1126/science.abd0875. ISSN 0036-8075. PMC 8185639. PMID 33833093.
- "Genecards QSER1".
- "NCBI PRR12 Gene".
- "Genomatix Tools: El Dorado".
- "NCBI GeoProfiles db; QSER1 GDS596".
- "NCBI EST Profile db; QSER1".
- Clarkson MR, Murphy M, Gupta S, Lambe T, Mackenzie HS, Godson C, Martin F, Brady HR (Mar 2002). "High glucose-altered gene expression in mesangial cells. Actin-regulatory protein gene expression is triggered by oxidative stress and cytoskeletal disassembly". The Journal of Biological Chemistry. 277 (12): 9707–12. doi:10.1074/jbc.M109172200. PMID 11784718.
- "NCBI GeoProfiles db; QSER1 GDS1891".
- Galindo CL, Skinner MA, Errami M, Olson LD, Watson DA, Li J, McCormick JF, McIver LJ, Kumar NM, Pham TQ, Garner HR (9 Dec 2009). "Transcriptional profile of isoproterenol-induced cardiomyopathy and comparison to exercise-induced cardiac hypertrophy and human cardiac failure". BMC Physiology. 9 (23): 23. doi:10.1186/1472-6793-9-23. PMC 2799380. PMID 20003209.
- "NCBI GeoProfiles db; QSER1 GDS3596".
- "NCBI GeoProfiles db; QSER1 GDS1746".
- "NCBI GeoProfiles db; QSER1 GDS3721".
- "NCBI GeoProfiles db; QSER1 GDS2810".
- "mfold".
- "SDSC Biology Workbench ClustalW".
- "NCBI QSER1 Protein".
- "SDSC Biology Work Bench; SAPS".
- "NCBI National Center for Biotechnology Information".
- "NCBI Conserved domains db; DUF4211".
- "NCBI Conserved domains db; PHA02939".
- "pSORT II Prediction".
- "SDSC Biology Workbench; PELE".
- "NCBI cBLAST; QSER1".
- "Phyre2".
- Möller A, Xie SQ, Hosp F, Lang B, Phatnani HP, James S, Ramirez F, Collin GB, Naggert JK, Babu MM, Greenleaf AL, Selbach M, Pombo A (Jun 2012). "Proteomic analysis of mitotic RNA polymerase II reveals novel interactors and association with proteins dysfunctional in disease". Molecular & Cellular Proteomics. 11 (6): M111.011767. doi:10.1074/mcp.M111.011767. PMC 3433901. PMID 22199231.
- Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER, Hurov KE, Luo J, Bakalarski CE, Zhao Z, et al. (May 2007). "ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage". Science. 316 (5828): 1160–6. Bibcode:2007Sci...316.1160M. doi:10.1126/science.1140321. PMID 17525332. S2CID 16648052.
- "ExPASy NetPhos".
- Tatham MH, Matic I, Mann M, Hay RT (21 Jun 2011). "Comparative proteomic analysis identifies a role for SUMO in protein quality control". Science Signaling. 4 (178): rs4. doi:10.1126/scisignal.2001484. PMID 21693764. S2CID 649212.
- Bruderer R, Tatham MH, Plechanovova A, Matic I, Garg AK, Hay RT (Feb 2011). "Purification and identification of endogenous polySUMO conjugates". EMBO Reports. 12 (2): 142–8. doi:10.1038/embor.2010.206. PMC 3049431. PMID 21252943.
- "ExPASy SUMOplot".
- Dombroski BA, Nayak RR, Ewens KG, Ankener W, Cheung VG, Spielman RS (May 2010). "Gene expression and genetic variation in response to endoplasmic reticulum stress in human cells" (PDF). American Journal of Human Genetics. 86 (5): 719–29. doi:10.1016/j.ajhg.2010.03.017. PMC 2869002. PMID 20398888. Archived from the original (PDF) on 2013-10-04. Retrieved 2013-05-02.
- Costa Y, Ding J, Theunissen TW, Faiola F, Hore TA, Shliaha PV, Fidalgo M, Saunders A, Lawrence M, Dietmann S, Das S, Levasseur DN, Li Z, Xu M, Reik W, Silva JC, Wang J (Mar 2013). "NANOG-dependent function of TET1 and TET2 in establishment of pluripotency". Nature. 495 (7441): 370–4. Bibcode:2013Natur.495..370C. doi:10.1038/nature11925. PMC 3606645. PMID 23395962.
- Kim W, Bennett EJ, Huttlin EL, Guo A, Li J, Possemato A, Sowa ME, Rad R, Rush J, Comb MJ, Harper JW, Gygi SP (Oct 2011). "Systematic and quantitative assessment of the ubiquitin-modified proteome". Molecular Cell. 44 (2): 325–40. doi:10.1016/j.molcel.2011.08.025. PMC 3200427. PMID 21906983.
- Danielsen JM, Sylvestersen KB, Bekker-Jensen S, Szklarczyk D, Poulsen JW, Horn H, Jensen LJ, Mailand N, Nielsen ML (Mar 2011). "Mass spectrometric analysis of lysine ubiquitylation reveals promiscuity at site level". Molecular & Cellular Proteomics. 10 (3): M110.003590. doi:10.1074/mcp.M110.003590. PMC 3047152. PMID 21139048.
- "NCBI AceView db; QSER1".
- "NCBI OMIM db; 11p13".
- "NCBI OMIM db; Exudative Vitreoretinopathy 3".
- "NCBI OMIM db; Candidiasis, Familial 3".
- "NCBI OMIM db; Centralopathic Epilepsy".
- "NCBI OMIM db; Autosomal Recessive Deafness 51".



