RDBP
Negative elongation factor E is a protein that in humans is encoded by the RDBP gene.[5]
Function
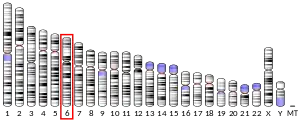
The protein encoded by this gene is part of a complex termed negative elongation factor (NELF) which represses RNA polymerase II transcript elongation. This protein bears similarity to nuclear RNA-binding proteins; however, it has not been demonstrated that this protein binds RNA. The protein contains a tract of alternating basic and acidic residues, largely arginine (R) and aspartic acid (D). The gene localizes to the major histocompatibility complex (MHC class III) region on chromosome 6.[5]
Interactions
RDBP has been shown to interact with:
References
- ENSG00000206357, ENSG00000231044, ENSG00000204356, ENSG00000206268, ENSG00000233801 GRCh38: Ensembl release 89: ENSG00000229363, ENSG00000206357, ENSG00000231044, ENSG00000204356, ENSG00000206268, ENSG00000233801 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000024369 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Entrez Gene: RDBP RD RNA binding protein".
- Narita T, Yamaguchi Y, Yano K, Sugimoto S, Chanarat S, Wada T, Kim DK, Hasegawa J, Omori M, Inukai N, Endoh M, Yamada T, Handa H (Mar 2003). "Human transcription elongation factor NELF: identification of novel subunits and reconstitution of the functionally active complex". Molecular and Cellular Biology. 23 (6): 1863–73. doi:10.1128/mcb.23.6.1863-1873.2003. PMC 149481. PMID 12612062.
- Lehner B, Semple JI, Brown SE, Counsell D, Campbell RD, Sanderson CM (Jan 2004). "Analysis of a high-throughput yeast two-hybrid system and its use to predict the function of intracellular proteins encoded within the human MHC class III region". Genomics. 83 (1): 153–67. doi:10.1016/s0888-7543(03)00235-0. PMID 14667819.
- Ewing RM, Chu P, Elisma F, Li H, Taylor P, Climie S, McBroom-Cerajewski L, Robinson MD, O'Connor L, Li M, Taylor R, Dharsee M, Ho Y, Heilbut A, Moore L, Zhang S, Ornatsky O, Bukhman YV, Ethier M, Sheng Y, Vasilescu J, Abu-Farha M, Lambert JP, Duewel HS, Stewart II, Kuehl B, Hogue K, Colwill K, Gladwish K, Muskat B, Kinach R, Adams SL, Moran MF, Morin GB, Topaloglou T, Figeys D (2007). "Large-scale mapping of human protein-protein interactions by mass spectrometry". Molecular Systems Biology. 3: 89. doi:10.1038/msb4100134. PMC 1847948. PMID 17353931.
Further reading
- Yu CY (1999). "Molecular genetics of the human MHC complement gene cluster". Experimental and Clinical Immunogenetics. 15 (4): 213–30. doi:10.1159/000019075. PMID 10072631. S2CID 25061446.
- Stevens M, De Clercq E, Balzarini J (Sep 2006). "The regulation of HIV-1 transcription: molecular targets for chemotherapeutic intervention". Medicinal Research Reviews. 26 (5): 595–625. doi:10.1002/med.20081. PMC 7168390. PMID 16838299.
- Surowy CS, Hoganson G, Gosink J, Strunk K, Spritz RA (Jun 1990). "The human RD protein is closely related to nuclear RNA-binding proteins and has been highly conserved". Gene. 90 (2): 299–302. doi:10.1016/0378-1119(90)90194-V. PMID 2119325.
- Speiser PW, White PC (Dec 1989). "Structure of the human RD gene: a highly conserved gene in the class III region of the major histocompatibility complex". DNA. 8 (10): 745–51. doi:10.1089/dna.1989.8.745. PMID 2612324.
- Lévi-Strauss M, Carroll MC, Steinmetz M, Meo T (Apr 1988). "A previously undetected MHC gene with an unusual periodic structure". Science. 240 (4849): 201–4. Bibcode:1988Sci...240..201L. doi:10.1126/science.3353717. PMID 3353717.
- Cheng J, Macon KJ, Volanakis JE (Sep 1993). "cDNA cloning and characterization of the protein encoded by RD, a gene located in the class III region of the human major histocompatibility complex". The Biochemical Journal. 294 (2): 589–93. doi:10.1042/bj2940589. PMC 1134496. PMID 8373374.
- Lee SG, Song K (Jun 1997). "Genomic organization of the human DDX13 gene located between RD and RP1 in the class III MHC complex". Molecules and Cells. 7 (3): 414–8. PMID 9264031.
- Büssow K, Cahill D, Nietfeld W, Bancroft D, Scherzinger E, Lehrach H, Walter G (Nov 1998). "A method for global protein expression and antibody screening on high-density filters of an arrayed cDNA library". Nucleic Acids Research. 26 (21): 5007–8. doi:10.1093/nar/26.21.5007. PMC 147919. PMID 9776767.
- Yang Z, Shen L, Dangel AW, Wu LC, Yu CY (Nov 1998). "Four ubiquitously expressed genes, RD (D6S45)-SKI2W (SKIV2L)-DOM3Z-RP1 (D6S60E), are present between complement component genes factor B and C4 in the class III region of the HLA". Genomics. 53 (3): 338–47. doi:10.1006/geno.1998.5499. PMID 9799600.
- Yamaguchi Y, Takagi T, Wada T, Yano K, Furuya A, Sugimoto S, Hasegawa J, Handa H (Apr 1999). "NELF, a multisubunit complex containing RD, cooperates with DSIF to repress RNA polymerase II elongation". Cell. 97 (1): 41–51. doi:10.1016/S0092-8674(00)80713-8. PMID 10199401. S2CID 16855257.
- Ping YH, Rana TM (Apr 2001). "DSIF and NELF interact with RNA polymerase II elongation complex and HIV-1 Tat stimulates P-TEFb-mediated phosphorylation of RNA polymerase II and DSIF during transcription elongation". The Journal of Biological Chemistry. 276 (16): 12951–8. doi:10.1074/jbc.M006130200. PMID 11112772.
- Yamaguchi Y, Inukai N, Narita T, Wada T, Handa H (May 2002). "Evidence that negative elongation factor represses transcription elongation through binding to a DRB sensitivity-inducing factor/RNA polymerase II complex and RNA". Molecular and Cellular Biology. 22 (9): 2918–27. doi:10.1128/MCB.22.9.2918-2927.2002. PMC 133766. PMID 11940650.
- Narita T, Yamaguchi Y, Yano K, Sugimoto S, Chanarat S, Wada T, Kim DK, Hasegawa J, Omori M, Inukai N, Endoh M, Yamada T, Handa H (Mar 2003). "Human transcription elongation factor NELF: identification of novel subunits and reconstitution of the functionally active complex". Molecular and Cellular Biology. 23 (6): 1863–73. doi:10.1128/MCB.23.6.1863-1873.2003. PMC 149481. PMID 12612062.
- Xie T, Rowen L, Aguado B, Ahearn ME, Madan A, Qin S, Campbell RD, Hood L (Dec 2003). "Analysis of the gene-dense major histocompatibility complex class III region and its comparison to mouse". Genome Research. 13 (12): 2621–36. doi:10.1101/gr.1736803. PMC 403804. PMID 14656967.
- Lehner B, Semple JI, Brown SE, Counsell D, Campbell RD, Sanderson CM (Jan 2004). "Analysis of a high-throughput yeast two-hybrid system and its use to predict the function of intracellular proteins encoded within the human MHC class III region". Genomics. 83 (1): 153–67. doi:10.1016/S0888-7543(03)00235-0. PMID 14667819.
- Beausoleil SA, Jedrychowski M, Schwartz D, Elias JE, Villén J, Li J, Cohn MA, Cantley LC, Gygi SP (Aug 2004). "Large-scale characterization of HeLa cell nuclear phosphoproteins". Proceedings of the National Academy of Sciences of the United States of America. 101 (33): 12130–5. Bibcode:2004PNAS..10112130B. doi:10.1073/pnas.0404720101. PMC 514446. PMID 15302935.
- Aiyar SE, Sun JL, Blair AL, Moskaluk CA, Lu YZ, Ye QN, Yamaguchi Y, Mukherjee A, Ren DM, Handa H, Li R (Sep 2004). "Attenuation of estrogen receptor alpha-mediated transcription through estrogen-stimulated recruitment of a negative elongation factor". Genes & Development. 18 (17): 2134–46. doi:10.1101/gad.1214104. PMC 515291. PMID 15342491.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.