RESF1
Retroelement silencing factor 1 is a protein that in humans is encoded by the RESF1 gene. RESF1 is broadly expressed in the lymph nodes, ovaries, appendix and spleen.[5] RESF1 shows characteristics of being a minor histocompatibility antigen, as well as tumor suppressor capabilities.[6][7] The high expression in the lymph nodes and spleen indicate function in the immune system.
| RESF1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | RESF1, C12orf35, UTA2-1, GET, retroelement silencing factor 1, KIAA1551 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | MGI: 1914496 HomoloGene: 19251 GeneCards: RESF1 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Gene
RESF1 is a protein coding gene found on Chromosome 12 and maps to 12p11.21.[8] Alternative names for this gene include Gonad Expressed Transcript (GET), UTA2-1 and C12orf35.[5] RESF1 has 7 exons, 3 of which occur before the start codon.[5]
Normal
A study of normal human tissue expression profiling shows that RESF1 is highly expressed in the thymus, spleen, bone marrow and liver.[9] This is interesting as it relates to common organs associated with the Immune system.
Gene tissue expression patterns found through the National Center for Biotechnology Information UniGene EST Profile showed that there was also high expression of RESF1 in the lymph nodes, uterus, mouth, thyroid, larynx and blood.[10]
Cancer
An evaluation of RESF1 expression in health states was performed using NCBI Unigene’s EST Profile.[11] Although RESF1 is highly expressed in uterine tumors, it is also highly expressed in the uterus, suggesting that it is unlikely the gene is associated closely with uterine cancer. However, RESF1 may be related to adrenal tumors, as there was lower expression of this gene within normal kidney tissue.
Transcription factor binding sites
Transcription factor binding sites within the promoter of RESF1 included mainly transcription factors that were associated with bone marrow cells, antibody- producing cells, and blood cells.[12] This supports the association of RESF1 with the functioning immune system.
Protein
RESF1 is 1747 amino acids in length and has one domain of unknown function, DUF4617.[13] The Molecular Weight of RESF1 is 194.9 kdal.[14] The basal isoelectric point is 8.95.[15] A localization prediction suggests that RESF1 is likely a nuclear protein.[15]
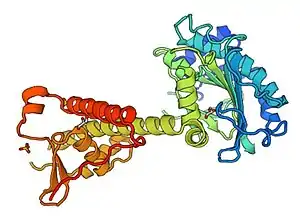
Protein structure
The secondary structure of RESF1 consists of mainly random coil structures (approximately 59.2%), few alpha helices (24% of residues) and fewer extended strands (15.8% of residues).[16]
A predicted 3-D structure was created using Swiss model work space, shown above.[17]
Protein interactions
RESF1 interacts with NANOG, MDM2, EXOC1 and CALML3. These interactions further suggest RESF1 is a nuclear protein, and that it may be associated with tumor-suppressor proteins and immune system proteins.[18]
EXOC1 was involved in a schizophrenia study, relating a schizophrenia risk gene (DISC1) to a network of protein-protein interactions.[19] This study used a two-hybrid assay as evidence to the protein interaction between RESF1 and EXOC1. EXOC1 functions as a response to microbial infections, which reduces viral RNA synthesis and protein translation.[20]
NANOG was predicted to interact with RESF1 based on an affinity capture-MS, which linked NANOG to proteins involved with the cell cycle. This study used affinity purification combined with high accuracy mass spectrometry to find specific protein interactions.[21] NANOG was also found to be an essential transcription factor in embryonic stem cells, specifically involved in gene expression to affect cell fate.[22]
MDM2 is a gene that interacts with others to affect the cell cycle and apoptosis, and is located in tissues common to RESF1, such as the uterus and lymph node.[23] MDM2 was found to interact with RESF1 through the use of a phage display library. This interaction further suggests that RESF1 is a nuclear protein, as MDM2 and its splice variants contain nuclear localization signals for nucleoplasmic distribution.[24]
CALML3 was found to interact with RESF1 based on affinity capture-MS assay, similar to how NANOG was found to interact with RESF1.[25] A study on CALML3 expression in epidermal development showed that CALML3 was useful marker for development, and loss of CALML3 expression correlated with malignant phenotypes.[26]
Evolutionary relationships
Orthologs
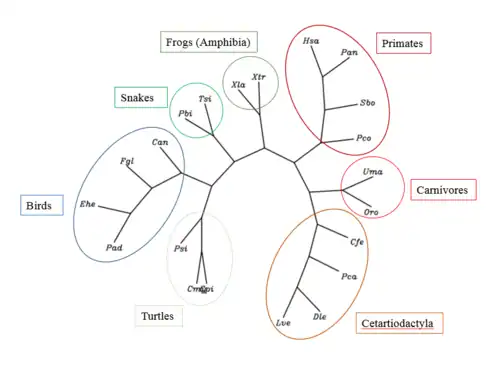
The closest orthologs to RESF1 are primates, however, conserved sequences can be found in whales, bears, snakes, birds, turtles, and frogs. Orthologs of RESF1 diverged as long ago as 353 million years ago (Xenopus laevis), while the closest evolutionary ortholog is Papio anubis, which diverged approximately 28.1 million years ago.
| Scientific Name | Name | Accession | Sequence Similarity % | Date of Divergence (MYA) |
|---|---|---|---|---|
| Papio anubis | Olive Baboon | XP_003906231.2 | 28.1 | 28.1 |
| Propithecus coquereli | Crowned Sifaka | XP_012496506 | 81 | 73 |
| Physeter Catadon | Sperm whale | XP_007116796.1 | 74 | 94 |
| Delphinapterus leucas | Beluga whale | XP_022433618.1 | 74 | 94 |
| Calypte anna | Anna's Hummingbird | XP_008493940 | 55 | 312 |
| Chrysemys picta belli | Western Painted Turtle | XP_008175485.1 | 43 | 312 |
| Python bivittatus | Burmese Python | XP_007443900.1 | 43 | 312 |
| Xenopus laevis | African Clawed Frog | XP_018107375 | 43 | 353 |
Phylogenetic tree
An unrooted phylogenetic tree of RESF1 was created of 20 orthologs and the human RESF1 gene.
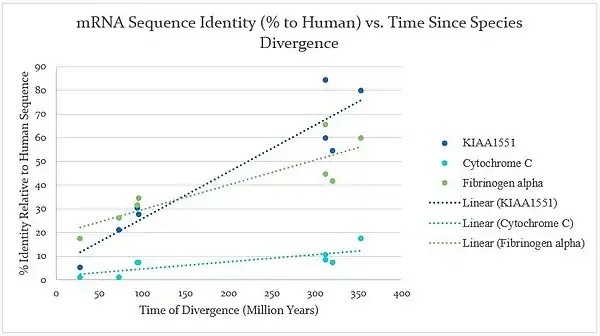
Molecular phylogeny
A graph shown below of the molecular evolution of RESF1 shows that it evolved relatively quickly compared to both cytochrome C, a slowly evolving protein, and fibrinogen alpha, which evolved more quickly than cytochrome C. The comparison shows that RESF1 is fairly quickly diverging, which suggests that it could be a gene that changes quickly in response to its environment, such as the introduction of a pathogen.
References
- GRCh38: Ensembl release 89: ENSG00000174718 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000032712 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "uncharacterized protein RESF1 [Homo sapiens] - Protein - NCBI". www.ncbi.nlm.nih.gov. Retrieved 2018-02-04.
- Roopenian D, Choi EY, Brown A (December 2002). "The immunogenomics of minor histocompatibility antigens". Immunological Reviews. 190: 86–94. doi:10.1034/j.1600-065x.2002.19007.x. PMID 12493008. S2CID 22243976.
- Oostvogels R, Minnema MC, van Elk M, Spaapen RM, te Raa GD, Giovannone B, Buijs A, van Baarle D, Kater AP, Griffioen M, Spierings E, Lokhorst HM, Mutis T (March 2013). "Towards effective and safe immunotherapy after allogeneic stem cell transplantation: identification of hematopoietic-specific minor histocompatibility antigen UTA2-1". Leukemia. 27 (3): 642–9. doi:10.1038/leu.2012.277. PMC 3593180. PMID 23079962.
- "RESF1 gene". Gene Cards.
- Yanai I, Benjamin H, Shmoish M, Chalifa-Caspi V, Shklar M, Ophir R, Bar-Even A, Horn-Saban S, Safran M, Domany E, Lancet D, Shmueli O (March 2005). "Genome-wide midrange transcription profiles reveal expression level relationships in human tissue specification". Bioinformatics. 21 (5): 650–9. doi:10.1093/bioinformatics/bti042. PMID 15388519.
- "Home - UniGene - NCBI". www.ncbi.nlm.nih.gov. Retrieved 2018-04-23.
- "Home - UniGene - NCBI". www.ncbi.nlm.nih.gov. Retrieved 2018-05-06.
- "ElDorado". Genomatix. Retrieved 18 April 2018.
- "Protein entry on RESF1". NCBI (National Center for Biotechnology Information).
- EMBL-EBI. "SAPS < Sequence Statistics < EMBL-EBI". www.ebi.ac.uk. Retrieved 2018-04-23.
- "Welcome to psort.org!!". psort.org. Retrieved 2018-04-23.
- Combet C, Blanchet C, Geourjon C, Deléage G (March 2000). "NPS@: network protein sequence analysis". Trends in Biochemical Sciences. 25 (3): 147–50. doi:10.1016/s0968-0004(99)01540-6. PMID 10694887.
- Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, Kiefer F, Gallo Cassarino T, Bertoni M, Bordoli L, Schwede T (July 2014). "SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information". Nucleic Acids Research. 42 (Web Server issue): W252–8. doi:10.1093/nar/gku340. PMC 4086089. PMID 24782522.
- "RESF1". BioGrid 3.4. Retrieved 19 April 2018.
- Camargo LM, Collura V, Rain JC, Mizuguchi K, Hermjakob H, Kerrien S, Bonnert TP, Whiting PJ, Brandon NJ (January 2007). "Disrupted in Schizophrenia 1 Interactome: evidence for the close connectivity of risk genes and a potential synaptic basis for schizophrenia". Molecular Psychiatry. 12 (1): 74–86. doi:10.1038/sj.mp.4001880. PMID 17043677.
- "EXOC1". Gene Cards. Retrieved 5 May 2018.
- Oliviero G, Munawar N, Watson A, Streubel G, Manning G, Bardwell V, Bracken AP, Cagney G (December 2015). "The variant Polycomb Repressor Complex 1 component PCGF1 interacts with a pluripotency sub-network that includes DPPA4, a regulator of embryogenesis". Scientific Reports. 5: 18388. Bibcode:2015NatSR...518388O. doi:10.1038/srep18388. PMC 4685312. PMID 26687479.
- Blinka S, Rao S (December 2017). "Nanog Expression in Embryonic Stem Cells - An Ideal Model System to Dissect Enhancer Function". BioEssays. 39 (12). doi:10.1002/bies.201700086. PMC 5878941. PMID 28977693.
- Guo Z, Wang X, Li H, Gao Y (2013). "Screening E3 substrates using a live phage display library". PLOS ONE. 8 (10): e76622. Bibcode:2013PLoSO...876622G. doi:10.1371/journal.pone.0076622. PMC 3790729. PMID 24124579.
- Schuster K, Fan L, Harris LC (April 2007). "MDM2 splice variants predominantly localize to the nucleoplasm mediated by a COOH-terminal nuclear localization signal". Molecular Cancer Research. 5 (4): 403–12. doi:10.1158/1541-7786.MCR-06-0146. PMID 17426254.
- Huttlin EL, Ting L, Bruckner RJ, Gebreab F, Gygi MP, Szpyt J, et al. (July 2015). "The BioPlex Network: A Systematic Exploration of the Human Interactome". Cell. 162 (2): 425–440. doi:10.1016/j.cell.2015.06.043. PMC 4617211. PMID 26186194.
- Bennett RD, Pittelkow MR, Strehler EE (2013). "Immunolocalization of the tumor-sensitive calmodulin-like protein CALML3 in normal human skin and hyperproliferative skin disorders". PLOS ONE. 8 (4): e62347. Bibcode:2013PLoSO...862347B. doi:10.1371/journal.pone.0062347. PMC 3630146. PMID 23638045.



