Red Sea brine pool microbiology
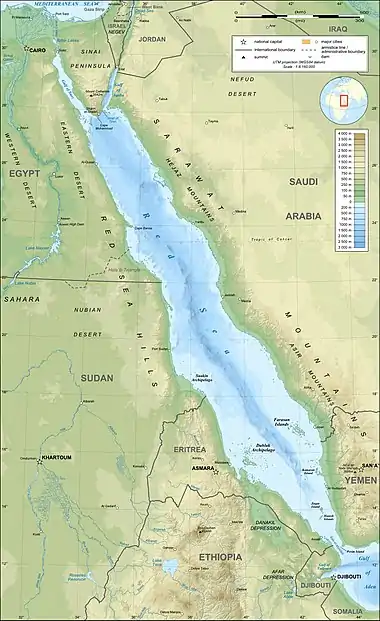
The Red Sea and its extensions of the Gulf of Suez and the Gulf of Aqaba contain the largest recorded concentration of deep sea brine pools on the planet. These pools have many features that make them un-inhabitable to almost all organisms on the planet, yet, certain communities of microbes thrive within these extreme environments that have temperature ranging from 2.0 °C all the way up to the high of 75 °C.[1] The Red Sea brine pools have extreme salinity concentrations and varying compositions of nutrients, chemicals properties and molecules that directly affect the microbiome between the estimated 25 individual pools in the region,[2][3] some of which are closely clustered together in groups leading to their undetermined classification of names. The brine pools in the region originate from hydrothermal vents and shifting of tectonic plates and the accumulation of water with properties that make it unsuitable for mixing leading to its accumulation within faults and divots in the sea floor. Atlantis Deep II, Discovery Deep and the Kebrit are the most investigated and researched brine pools among the many located within the Red Sea[4] Additionally, many microbial species form beneficial symbiotic relationships with organisms living and feeding in proximity to the pools. These relationships allow for the study of specialised adaptations of microbes to brine pool environments.
List
In addition to the originally-discovered warm brine pools, recent discoveries have found four smaller warm brine pools named the NEOM Brine Pools located in the Gulf of Aqaba. Furthermore, multiple cold seeps have been identified in the region of the Red Sea (Thuwal Cold Seeps) consisting of two individual pools. Three of these Red Sea brine pools are unnamed as they are small and potentially extensions of other nearby larger pools.
| Brine pool number | Warm brine pool | Cold seeps |
|---|---|---|
| 1 | Albatross Deep | |
| 2 | Atlantis II Deep | |
| 3 | Chain Deep | |
| 4 | Conrad Deep | |
| 5 | Discovery Deep | |
| 6 | Erba Deep | |
| 7 | Kebrit Deep | |
| 8, 9, 10, 11, | NEOM brine pools | |
| 12 | Nereus Deep | |
| 13 | Oceanographers Deep | |
| 14 | Port Sudan Deep | |
| 15 | Shaban Deep | |
| 16 | Shagara Deep | |
| 17 | Suakin Deep | |
| 18 | Valdiva Deeps | |
| 19 | Wando Basin | |
| 20, 21 | Thuwal Cold Seeps |
Viral diversity
Composition
The virus community within the many Red Sea brine pools is largely unexplored. However, with the use of metagenomics, viral communities of the Atlantic II Deep (ADII), Discovery Deep (DD) and the Kebrit Deep (KD) reveal diverse and distinct viruses within and between the brine pools. Across all three brine pools, double-stranded DNA (dsDNA) are the most dominant viruses.[5][6] Of the dsDNA viruses investigated, Caudovirales are the most abundant across all three brine pools. Low abundances of Phycodnaviridae, and trace amounts of Iridoviridae are also present within the brine-seawater interfaces, and thus may be indicative of a "pickling" effect rather than a host-specific presence.[5]
Stratification of viral communities
Viral species tend to follow their bacterial-host population dynamics. Bacterial and archaeal composition and abundance differ between specific layers of the brine pool including overlying brine seawater, brine-water interface, brine-pool sediments, and direct brine waters.[7][8][9] As a result, the viral community within the brine pools of the Red Sea are stratified across the brine to seawater interface.[10] The Kebrit Deep's brine-seawater interface upper layer is dominated by marine bacteria infecting viruses, relative to the lower layer brine-seawater interface which is dominated by haloviruses and halophages[5]
Role of viruses
Deep sea marine viruses maintain the diversity and abundance of the microbial community, recycling and supplying essential nutrients and biomolecules, and regulating the biogeochemical cycling.[11][12][13][14] In deep, anoxic environments such as the Red Sea brine pools, viral infection of prokaryotes releases cellular DNA. Extracellular DNA released through infection supplies highly labile biomolecules in these water conditions limited by external input supporting microbial communities.[13] Through lysogenic viral infection and horizontal gene transfer, the viral community in the Red Sea brine pools contribute to microbial DNA repair, nucleotide metabolism,[15] as well as the evolutionary adaptations of the microbial community.[6][15]
Bacterial and archaeal diversity and adaptations
The Red Sea brine pools were once thought to be inhospitable to life.[7] However, extremophiles have adapted to these environments through the development of novel enzymes and metabolic pathways.[16][4][17]
The various brine pools contain somewhat similar diversity of microbes, however due to different characteristics of each brine pool, a distinct microbe composition is seen. Similar to the Gulf of Mexico[18] brine pools, the Red Sea brine pool experiences stratification within each distinct brine pool.[19] Therefore, as a result of the stratification, varying physical and chemical properties occur with respect to depth ensuing a transition in the microbial community with respect to depth.[16][7]
Moreover, the stratification causes sharp brine-seawater interfaces, with typically steep gradients salinity, temperature, density, oxygen, and pH gradients. These distinct interfaces between two layers of well mixed water are characteristic of liquids that are stabilised by salt but destabilised by heating from below. Heat at the bottom of these stable salinity gradients causes double-diffusive convection events.[1]
Specific bacterial composition
Deep-sea anoxic brines (referred to as DHABs, deep hypersaline anoxic basins) are developed by a process of re-dissolving of evaporitic sediments buried at shallow depths, tectonic ejection of the interstitial brine reacted with the evaporites and/or by hydrothermal phase separation.[20]
There are examples of various types of bacteria (Table 1) under the brine pools.[21]
| Class | Family | Genus/species/strain |
| Gammaproteobacteria | Pseudomonadaceae | Pseudomonas sp |
| Deltaproteobacteria | Desulfovibrionaceae | Desulfovibrio sp. |
| Deferribacteres | Deferrribacteraceae | Flexistipes sinusarabici |
| Gammaproteobacteria | Alteromonadaceae | Marinobacter salsuginis |
| Clostridia | Halanaerobiaceae | Halanaerobium sp. |
| Firmicutes/Mollicutes | Haloplasmataceae | Haloplasma contractile |
| Halobacteria | Halobacteriaceae | Halorhabdus tiamatea |
| Gammaproteobacteria | Alteromonadaceae | Marinobacter salsuginis |
| Colwelliaceae | Salinisphaera shabanensis | |
| Idiomarinaceae | Halanaerobium sp. | |
| ‘Salinisphaeraceae’ | Nitrosovibrio sp. |
Influence of stratification
Stratification within and surrounding water layers is a characteristic of brine pools due to the highly saline environment. Specifically, in the Red Sea, as a result of this stratification in the deep sea brine pools, microbial communities are subject to differences their vertical distribution and composition.[22] For example, through the use of metagenomics and pyrosequencing, two deeps; the Atlantic II Deep and the Discovery Deep's microbial communities were investigated with respect to vertical distribution. In terms of archaeal communities, both deeps showed similar composition having the upper layer (20–50 m) enriched in Halobacteriales, and as salt concentration increased and oxygen decreased, Desulfurococcales tended to dominate due to physiological adaptations.[22][23] The bacterial composition in the upper layer consisted of Cyanobacteria due to the presence of light. Deeper in the water column, Proteobacteria, specifically the gamma-subdivision group (orders Thiotrichales, Salinisphaerales, Chromatiales and Alteromonadales) were found to dominate the more extreme conditions.[22]
The stratification within the Red Sea Brine Pools therefore allows for a complex composition of the microbial community with depth. Due to the variability between each Red Sea Brine Pool, this would account for differences in taxa at each location and at each depth.
Bacterial enzymes
Extremozymes are very prominent in Red Sea Brine Pools as they have the ability to be able to catalyse reactions under harsh environments.[24]
In general, extremozymes can be separated into categories depending on habitats, such as those that can resist extremes of cold (psychrophilic), heat (thermophilic and hyperthermophilic), acidity (halophilic), alkalinity (halophilic), and salinity (halophilic).[25] Red Sea Brine Pools are subject to host a polyextremophilic microbiological community providing the environment with a source of extremozymes.
Moreover, most of the extremozymes are classified into three classes of enzymes which are oxidoreductases, transferases, and hydrolases,[21] which are important in terms of metabolic processes for the organisms within this habitat as well as poses for potential application uses.[4]
Symbiotic Relationships
Several anoxic, high salinity deep-sea water basins in the Red Sea generate notably sharp interfaces that are referred to a variety of physicochemical gradients.[26] By acting as a particle trap for organic and inorganic elements from saltwater, brine pools have the ability to significantly increase the supply of nutrients and the possibility for bacterial growth.[27] On the other hand, halophilic bacteria are required to evolve specific structures to resist in the environmental condition of brine pools habitat. For example, halophilic enzymes have a higher proportion of acidic amino acid residues than non-halophilic homologues. In the meanwhile, these bacterias accumate high concentrations of KCl in their cytoplasm which reach saturation.[28]
Potential applications for enzymes
As of recent years, twelve enzymes have been detected in the Red Sea brine pools (Atlantis Deep II, Discovery Deep, and Kebrit Deep) with specific biochemical properties that are promising in their potential application in multiple disciplinaries.[4] The microbes that inhabit the hypersaline, high temperature, low oxygen, and high concentrations of toxic metal Red Sea brine pools produce and/or accumulate microbial enzymes known as extremozymes allowing life to sustain.[29] The chemical and physical properties, in addition to the stability of the extremozymes provides potential use in areas including but limited to, industrial, biotechnical, and pharmaceutical disciplinaries.[4][30][31]
The different enzymes can be attributed to the different organisms that live within each brine pool due to the environments variable conditions. The Kebrit Deep one of the smallest brine pools among the others located in the Red Sea and is not considered a hot brine (21-23 °C).[4] Other characteristics include a pH of 5.2, 84 m thick brine layer, and is rich in hydrogen sulfide.[8][32] The Atlantic Deep II is among the largest Red Sea Brine pool with characteristics of high temperatures (~68 °C), pH of 5.3, and high metal content.[33][34] Whereas the Discovery Deep is similar to that of the Atlantic II Deep, however has differences in metal content and less extreme conditions.[35][36]
| Brine Pool | Extremozyme | Potential Use |
|---|---|---|
| Atlantic Deep II | ADH/A1a[4] | Pharmaceutical and Biodegradation[4][37] |
| ATII-TrxR[38] | Cancer therapy and Antibiotics[39][4] | |
| ATII-LCL MerA[40][41] | Bioremediation and Mercury detoxification[4][41] | |
| ATII-LCL-NH[40] | Bioremediation[4] | |
| BR3 pol[42] | Biomedical DNA techniques[4][43] | |
| ATII-APH(3')[44] | Biotechnology and Antibiotics[4][45] | |
| EstATII[46] | Pharmaceuticals, Cosmetics, and Biodegradation[47][4] | |
| ATII-ABL[48] | Biotechnology and Antibiotics[4][45] | |
| NItraS-ATII[49] | Pharmaceuticals and Bioremediation[4] | |
| Discovery Deep | ADH/D1[50] | Pharmaceutical and Biodegradation[4] |
| CA_D[51] | Carbon Sequestration[4][52] | |
| Kebrit Deep | K09H MerA[53] | Bioremediation and Mercury detoxification[4][41] |
| K35NH MerA[53] | Bioremediation and Mercury detoxification[4][41] |
Recent discoveries and future implications
The Thuwal cold seeps were accidentally discovered in the Red Sea at about 850m depth on May 7, 2010 by a remotely operated vehicle (ROV).[54] The team of scientists were conducting a continental slope survey of the Red Sea as part of the KAUST Red Sea Expedition 2010.[54] These cold seeps occur along the tectonically active and passive continental margin within the Red Sea where hypersaline brine seeps out of the seabed where they associate with brine pool formations.[54] The Thuwal cold seeps are referred to as ‘cold’ due to their cooler temperature (about 21.7 °C) in relation to other brine pools found within the Red Sea.
Cold seeps are a very important component of deep sea ecosystems where chemosynthetic bacteria acting as the base of this community is using the methane and hydrogen sulphide in seep water as their energy source.[55] The microbial community acts as a base of the food chain for a very unique ecosystem of organisms that helps sustain and feed bottom and filter feeders such as bivalves.
Discovery of NEOM Brine Pools
During a 2020 research expedition, with the use of bathymetry and geophysical observations, four complex brine pools were recently discovered in the northern Gulf of Aqaba of the Red Sea which has not been found to harbour brine pools thus far. The discovery consisted of three small brine pools less than 10 m2 and another pool that was 10,000 m2 which were given the name NEOM Brine Pools.[31] NEOM Brine Pools are unique from other Red Sea Brine Pools as they are located in much closer proximity to the shore. Due to the brine pools location of two km off-shore, they are subject to sediment shed and as a result can preserve geophysical properties that could potentially give insight to historical tsunamis, flash floods and earthquakes that may have occurred in the Gulf Aqaba.[31]
Within these NEOM Brine Pools similar to other Red Sea Brine Pools, stratification of the overlaying water, the interface, and the brine water caused stratification of microbial diversity.[31] The upper layer consisted of aerobic microbes such as Gammaproteobacteria, Thaumarchaeota Alphaproteobacteria, and Nitrospira. In the deeper convective layers of the NEOM pools, sulfate-reducing , methanogens, and reducing microorganisms were more abundant given anaerobic conditions.[31]
References
- Antunes, André; Ngugi, David Kamanda; Stingl, Ulrich (2011-05-30). "Microbiology of the Red Sea (and other) deep-sea anoxic brine lakes". Environmental Microbiology Reports. 3 (4): 416–433. doi:10.1111/j.1758-2229.2011.00264.x. ISSN 1758-2229. PMID 23761304.
- "Metalliferous Sediments of the Red Sea", Metalliferous Sediments of the World Ocean, Berlin/Heidelberg: Springer-Verlag, pp. 127–210, 2006, doi:10.1007/3-540-30969-1_3, ISBN 3-540-27869-9, retrieved 2023-03-16
- Antunes, André; Ngugi, David Kamanda; Stingl, Ulrich (August 2011). "Microbiology of the Red Sea (and other) deep-sea anoxic brine lakes: The deep-sea brines of the Red Sea". Environmental Microbiology Reports. 3 (4): 416–433. doi:10.1111/j.1758-2229.2011.00264.x. PMID 23761304.
- Renn, Dominik; Shepard, Lera; Vancea, Alexandra; Karan, Ram; Arold, Stefan T.; Rueping, Magnus (2021-10-27). "Novel Enzymes From the Red Sea Brine Pools: Current State and Potential". Frontiers in Microbiology. 12: 732856. doi:10.3389/fmicb.2021.732856. ISSN 1664-302X. PMC 8578733. PMID 34777282.
- Antunes, André; Alam, Intikhab; Simões, Marta Filipa; Daniels, Camille; Ferreira, Ari J. S.; Siam, Rania; El-Dorry, Hamza; Bajic, Vladimir B. (2015-10-01). "First Insights into the Viral Communities of the Deep-sea Anoxic Brines of the Red Sea". Genomics, Proteomics & Bioinformatics. SI: Metagenomics of Marine Environments. 13 (5): 304–309. doi:10.1016/j.gpb.2015.06.004. ISSN 1672-0229. PMC 4678784. PMID 26529193. S2CID 17451269.
- Aziz, S. (2017).Virome of red sea brine pools and other hydrothermal vents [Master's Thesis, the American University in Cairo]. AUC Knowledge Fountain. https://fount.aucegypt.edu/etds/642
- Bougouffa, S.; Yang, J. K.; Lee, O. O.; Wang, Y.; Batang, Z.; Al-Suwailem, A.; Qian, P. Y. (June 2013). "Distinctive Microbial Community Structure in Highly Stratified Deep-Sea Brine Water Columns". Applied and Environmental Microbiology. 79 (11): 3425–3437. Bibcode:2013ApEnM..79.3425B. doi:10.1128/aem.00254-13. ISSN 0099-2240. PMC 3648036. PMID 23542623.
- Eder, Wolfgang; Jahnke, Linda L.; Schmidt, Mark; Huber, Robert (July 2001). "Microbial Diversity of the Brine-Seawater Interface of the Kebrit Deep, Red Sea, Studied via 16S rRNA Gene Sequences and Cultivation Methods". Applied and Environmental Microbiology. 67 (7): 3077–3085. Bibcode:2001ApEnM..67.3077E. doi:10.1128/aem.67.7.3077-3085.2001. ISSN 0099-2240. PMC 92984. PMID 11425725.
- Qian, Pei-Yuan; Wang, Yong; Lee, On On; Lau, Stanley C K; Yang, Jiangke; Lafi, Feras F; Al-Suwailem, Abdulaziz; Wong, Tim YH (2010-07-29). "Vertical stratification of microbial communities in the Red Sea revealed by 16S rDNA pyrosequencing". The ISME Journal. 5 (3): 507–518. doi:10.1038/ismej.2010.112. ISSN 1751-7362. PMC 3105721. PMID 20668490. S2CID 13199360.
- Antunes, André; Kaartvedt, Stein; Schmidt, Mark (2019), Rasul, Najeeb M.A.; Stewart, Ian C.F. (eds.), "Geochemistry and Life at the Interfaces of Brine-Filled Deeps in the Red Sea", Oceanographic and Biological Aspects of the Red Sea, Cham: Springer International Publishing, pp. 185–194, doi:10.1007/978-3-319-99417-8_11, ISBN 978-3-319-99417-8, S2CID 133581777, retrieved 2023-03-15
- Thomas, Elaina; Anderson, Rika E.; Li, Viola; Rogan, L. Jenni; Huber, Julie A. (2021-06-29). Petersen, Jillian Michelle (ed.). Simon Roux. "Diverse Viruses in Deep-Sea Hydrothermal Vent Fluids Have Restricted Dispersal across Ocean Basins". mSystems. 6 (3): e00068–21. doi:10.1128/mSystems.00068-21. ISSN 2379-5077. PMC 8269205. PMID 34156293.
- Cheng, Ruolin; Li, Xiaofeng; Jiang, Lijing; Gong, Linfeng; Geslin, Claire; Shao, Zongze (2022-12-24). "Virus diversity and interactions with hosts in deep-sea hydrothermal vents". Microbiome. 10 (1): 235. doi:10.1186/s40168-022-01441-6. ISSN 2049-2618. PMC 9789665. PMID 36566239. S2CID 255096003.
- Corinaldesi, Cinzia; Dell'Anno, Antonio; Danovaro, Roberto (March 2007). "Viral infection plays a key role in extracellular DNA dynamics in marine anoxic systems". Limnology and Oceanography. 52 (2): 508–516. Bibcode:2007LimOc..52..508C. doi:10.4319/lo.2007.52.2.0508. ISSN 0024-3590. S2CID 85601366.
- De Corte, Daniele; Martínez, Joaquín Martínez; Cretoiu, Mariana Silvia; Takaki, Yoshihiro; Nunoura, Takuro; Sintes, Eva; Herndl, Gerhard J.; Yokokawa, Taichi (2019-08-21). "Viral Communities in the Global Deep Ocean Conveyor Belt Assessed by Targeted Viromics". Frontiers in Microbiology. 10: 1801. doi:10.3389/fmicb.2019.01801. ISSN 1664-302X. PMC 6712177. PMID 31496997.
- Adel, Mustafa; Elbehery, Ali H. A.; Aziz, Sherry K.; Aziz, Ramy K.; Grossart, Hans-Peter; Siam, Rania (2016-09-06). "Viruses-to-mobile genetic elements skew in the deep Atlantis II brine pool sediments". Scientific Reports. 6 (1): 32704. Bibcode:2016NatSR...632704A. doi:10.1038/srep32704. ISSN 2045-2322. PMC 5011723. PMID 27596223.
- Behzad, Hayedeh; Ibarra, Martin Augusto; Mineta, Katsuhiko; Gojobori, Takashi (February 2016). "Metagenomic studies of the Red Sea". Gene. 576 (2): 717–723. doi:10.1016/j.gene.2015.10.034. hdl:10754/581498. ISSN 0378-1119. PMID 26526132.
- Wang, Yong; Cao, Huiluo; Zhang, Guishan; Bougouffa, Salim; Lee, On On; Al-Suwailem, Abdulaziz; Qian, Pei-Yuan (2013-04-29). "Autotrophic Microbe Metagenomes and Metabolic Pathways Differentiate Adjacent Red Sea Brine Pools". Scientific Reports. 3 (1): 1748. Bibcode:2013NatSR...3E1748W. doi:10.1038/srep01748. ISSN 2045-2322. PMC 3638166. PMID 23624511.
- Ian R. MacDonald (1996). "Thermal and Density Stratification in a Seafloor Brine Pool, Northern Gulf of Mexico: ABSTRACT". AAPG Bulletin. 80. doi:10.1306/522b3353-1727-11d7-8645000102c1865d. ISSN 0149-1423.
- Blanc, Gérard; Anschutz, Pierre (1995). <0543:nsithb>2.3.co;2 "New stratification in the hydrothermal brine system of the Atlantis II Deep, Red Sea". Geology. 23 (6): 543. Bibcode:1995Geo....23..543B. doi:10.1130/0091-7613(1995)023<0543:nsithb>2.3.co;2. ISSN 0091-7613.
- Cita, M.B. (2006) Exhumation of Messinian evaporites in the deep-sea and creation of deep anoxic brine-filled collapsed basins. Sediment Geol 188–189: 357–378.
- Renn, D., Shepard, L., Vancea, A., Karan, R., Arold, S. T., & Rueping, M. (2021). Novel enzymes from the red sea brine pools: current state and potential. Frontiers in Microbiology, 12, 732856.
- Qian, Pei-Yuan; Wang, Yong; Lee, On On; Lau, Stanley C. K.; Yang, Jiangke; Lafi, Feras F.; Al-Suwailem, Abdulaziz; Wong, Tim YH (March 2011). "Vertical stratification of microbial communities in the Red Sea revealed by 16S rDNA pyrosequencing". The ISME Journal. 5 (3): 507–518. doi:10.1038/ismej.2010.112. ISSN 1751-7370. PMC 3105721. PMID 20668490.
- Siam, Rania; Mustafa, Ghada A.; Sharaf, Hazem; Moustafa, Ahmed; Ramadan, Adham R.; Antunes, Andre; Bajic, Vladimir B.; Stingl, Uli; Marsis, Nardine G. R.; Coolen, Marco J. L.; Sogin, Mitchell; Ferreira, Ari J. S.; Dorry, Hamza El (2012-08-20). "Unique Prokaryotic Consortia in Geochemically Distinct Sediments from Red Sea Atlantis II and Discovery Deep Brine Pools". PLOS ONE. 7 (8): e42872. Bibcode:2012PLoSO...742872S. doi:10.1371/journal.pone.0042872. ISSN 1932-6203. PMC 3423430. PMID 22916172.
- Dumorne, K., Cordova, D. C., Astorga-Elo, M., and Renganathan, P. (2017). Extremozymes: a potential source for industrial applications. J. Microbiol. Biotechnol. 27, 649–659.
- Sarmiento, F., Peralta, R., and Blamey, J. M. (2015). Cold and hot extremozymes: industrial relevance and current trends. Front. Bioeng. Biotechnol. 3:148. doi:10.3389/fbioe.2015.00148
- Antunes, A., Ngugi, D. K., & Stingl, U. (2011). Microbiology of the Red Sea (and other) deep‐sea anoxic brine lakes. Environmental microbiology reports, 3(4), 416-433.
- Eder, W. (2000) Nachweis, Isolierung Und Charakterisierung Extremophiler Mikro-Organismen Aus Hydrothermalgebieten (Ph.D Thesis). Regensburg, Germany: Lehrstuhl für Mikrobiologie, Universität Regensburg.
- Madern, D., Ebel, C., & Zaccai, G. (2000). Halophilic adaptation of enzymes. Extremophiles, 4, 91-98.
- Akal, Anastassja L.; Karan, Ram; Hohl, Adrian; Alam, Intikhab; Vogler, Malvina; Grötzinger, Stefan W.; Eppinger, Jörg; Rueping, Magnus (February 2019). "A polyextremophilic alcohol dehydrogenase from the Atlantis II Deep Red Sea brine pool". FEBS Open Bio. 9 (2): 194–205. doi:10.1002/2211-5463.12557. ISSN 2211-5463. PMC 6356862. PMID 30761247.
- Dumorne, Kelly; Cordova, David Camacho; Astorga-Elo, Marcia; Renganathan, Prabhaharan (2017-04-28). "Extremozymes: A Potential Source for Industrial Applications". Journal of Microbiology and Biotechnology. 27 (4): 649–659. doi:10.4014/jmb.1611.11006. ISSN 1017-7825. PMID 28104900.
- Purkis, Sam J.; Shernisky, Hannah; Swart, Peter K.; Sharifi, Arash; Oehlert, Amanda; Marchese, Fabio; Benzoni, Francesca; Chimienti, Giovanni; Duchâtellier, Gaëlle; Klaus, James; Eberli, Gregor P.; Peterson, Larry; Craig, Andrew; Rodrigue, Mattie; Titschack, Jürgen (2022-06-27). "Discovery of the deep-sea NEOM Brine Pools in the Gulf of Aqaba, Red Sea". Communications Earth & Environment. 3 (1): 146. Bibcode:2022ComEE...3..146P. doi:10.1038/s43247-022-00482-x. ISSN 2662-4435. S2CID 250065344.
- Schmidt, M.; Botz, R.; Faber, E.; Schmitt, M.; Poggenburg, J.; Garbe-Schönberg, D.; Stoffers, P. (October 2003). "High-resolution methane profiles across anoxic brine–seawater boundaries in the Atlantis-II, Discovery, and Kebrit Deeps (Red Sea)". Chemical Geology. 200 (3–4): 359–375. Bibcode:2003ChGeo.200..359S. doi:10.1016/s0009-2541(03)00206-7. ISSN 0009-2541.
- Anschutz, Pierre; Blanc, Gérard (July 1996). "Heat and salt fluxes in the Atlantis II Deep (Red Sea)". Earth and Planetary Science Letters. 142 (1–2): 147–159. Bibcode:1996E&PSL.142..147A. doi:10.1016/0012-821x(96)00098-2. ISSN 0012-821X.
- Danielsson, Lars-Göran; Dyrssen, David; Granéli, Anders (December 1980). "Chemical investigations of Atlantis II and discovery brines in the Red Sea". Geochimica et Cosmochimica Acta. 44 (12): 2051–2065. Bibcode:1980GeCoA..44.2051D. doi:10.1016/0016-7037(80)90203-3. ISSN 0016-7037.
- Abdallah, Rehab Z.; Adel, Mustafa; Ouf, Amged; Sayed, Ahmed; Ghazy, Mohamed A.; Alam, Intikhab; Essack, Magbubah; Lafi, Feras F.; Bajic, Vladimir B.; El-Dorry, Hamza; Siam, Rania (2014-09-23). "Aerobic methanotrophic communities at the Red Sea brine-seawater interface". Frontiers in Microbiology. 5: 487. doi:10.3389/fmicb.2014.00487. ISSN 1664-302X. PMC 4172156. PMID 25295031.
- Antunes, André; Ngugi, David Kamanda; Stingl, Ulrich (August 2011). "Microbiology of the Red Sea (and other) deep-sea anoxic brine lakes: The deep-sea brines of the Red Sea". Environmental Microbiology Reports. 3 (4): 416–433. doi:10.1111/j.1758-2229.2011.00264.x. PMID 23761304.
- Atalah, Joaquín; Cáceres-Moreno, Paulina; Espina, Giannina; Blamey, Jenny M. (May 2019). "Thermophiles and the applications of their enzymes as new biocatalysts". Bioresource Technology. 280: 478–488. doi:10.1016/j.biortech.2019.02.008. ISSN 0960-8524. PMID 30826176. S2CID 73509194.
- Badiea, Elham A.; Sayed, Ahmed A.; Maged, Mohamad; Fouad, Walid M.; Said, Mahmoud M.; Esmat, Amr Y. (2019-05-31). "A novel thermostable and halophilic thioredoxin reductase from the Red Sea Atlantis II hot brine pool". PLOS ONE. 14 (5): e0217565. Bibcode:2019PLoSO..1417565B. doi:10.1371/journal.pone.0217565. ISSN 1932-6203. PMC 6544261. PMID 31150456.
- Harbut, Michael B.; Vilchèze, Catherine; Luo, Xiaozhou; Hensler, Mary E.; Guo, Hui; Yang, Baiyuan; Chatterjee, Arnab K.; Nizet, Victor; Jacobs, William R.; Schultz, Peter G.; Wang, Feng (2015-04-07). "Auranofin exerts broad-spectrum bactericidal activities by targeting thiol-redox homeostasis". Proceedings of the National Academy of Sciences. 112 (14): 4453–4458. Bibcode:2015PNAS..112.4453H. doi:10.1073/pnas.1504022112. ISSN 0027-8424. PMC 4394260. PMID 25831516.
- Maged, Mohamad; El Hosseiny, Ahmed; Saadeldin, Mona Kamal; Aziz, Ramy K.; Ramadan, Eman (February 2019). "Thermal Stability of a Mercuric Reductase from the Red Sea Atlantis II Hot Brine Environment as Analyzed by Site-Directed Mutagenesis". Applied and Environmental Microbiology. 85 (3). Bibcode:2019ApEnM..85E2387M. doi:10.1128/aem.02387-18. ISSN 0099-2240. PMC 6344611. PMID 30446558.
- Sayed, Ahmed; Ghazy, Mohamed A.; Ferreira, Ari J.S.; Setubal, João C.; Chambergo, Felipe S.; Ouf, Amged; Adel, Mustafa; Dawe, Adam S.; Archer, John A.C.; Bajic, Vladimir B.; Siam, Rania; El-Dorry, Hamza (January 2014). "A Novel Mercuric Reductase from the Unique Deep Brine Environment of Atlantis II in the Red Sea". Journal of Biological Chemistry. 289 (3): 1675–1687. doi:10.1074/jbc.M113.493429. PMC 3894346. PMID 24280218.
- Hamdan, S., and Takahashi, M. (2015). DNA Polymerazes from the Red Sea Brine Pool Organisms. Patent No. WO 2015166354 A2. Thuwal: King Abdullah University of Science and Technology
- Gong, Jin-Song; Lu, Zhen-Ming; Li, Heng; Shi, Jin-Song; Zhou, Zhe-Min; Xu, Zheng-Hong (December 2012). "Nitrilases in nitrile biocatalysis: recent progress and forthcoming research". Microbial Cell Factories. 11 (1): 142. doi:10.1186/1475-2859-11-142. ISSN 1475-2859. PMC 3537687. PMID 23106943.
- Takahashi, Masateru; Takahashi, Etsuko; Joudeh, Luay I.; Marini, Monica; Das, Gobind; Elshenawy, Mohamed M.; Akal, Anastassja; Sakashita, Kosuke; Alam, Intikhab; Tehseen, Muhammad; Sobhy, Mohamed A.; Stingl, Ulrich; Merzaban, Jasmeen S.; Di Fabrizio, Enzo; Hamdan, Samir M. (June 2018). "Dynamic structure mediates halophilic adaptation of a DNA polymerase from the deep‐sea brines of the Red Sea". The FASEB Journal. 32 (6): 3346–3360. doi:10.1096/fj.201700862RR. ISSN 0892-6638. PMC 6051491. PMID 29401622.
- Terekhov, Stanislav S.; Mokrushina, Yuliana A.; Nazarov, Anton S.; Zlobin, Alexander; Zalevsky, Arthur; Bourenkov, Gleb; Golovin, Andrey; Belogurov, Alexey; Osterman, Ilya A.; Kulikova, Alexandra A.; Mitkevich, Vladimir A.; Lou, Hua Jane; Turk, Benjamin E.; Wilmanns, Matthias; Smirnov, Ivan V. (2020-06-26). "A kinase bioscavenger provides antibiotic resistance by extremely tight substrate binding". Science Advances. 6 (26): eaaz9861. Bibcode:2020SciA....6.9861T. doi:10.1126/sciadv.aaz9861. ISSN 2375-2548. PMC 7314540. PMID 32637600.
- Mohamed, Yasmine M.; Ghazy, Mohamed A.; Sayed, Ahmed; Ouf, Amged; El-Dorry, Hamza; Siam, Rania (2013-11-28). "Isolation and characterization of a heavy metal-resistant, thermophilic esterase from a Red Sea Brine Pool". Scientific Reports. 3 (1): 3358. Bibcode:2013NatSR...3E3358M. doi:10.1038/srep03358. ISSN 2045-2322. PMC 6506439. PMID 24285146.
- Panda, T.; Gowrishankar, B. S. (April 2005). "Production and applications of esterases". Applied Microbiology and Biotechnology. 67 (2): 160–169. doi:10.1007/s00253-004-1840-y. ISSN 0175-7598. PMID 15630579. S2CID 33489838.
- Elbehery, Ali H. A.; Leak, David J.; Siam, Rania (January 2017). "Novel thermostable antibiotic resistance enzymes from the Atlantis II Deep Red Sea brine pool". Microbial Biotechnology. 10 (1): 189–202. doi:10.1111/1751-7915.12468. PMC 5270753. PMID 28004885.
- Sonbol, Sarah A.; Ferreira, Ari J. S.; Siam, Rania (December 2016). "Red Sea Atlantis II brine pool nitrilase with unique thermostability profile and heavy metal tolerance". BMC Biotechnology. 16 (1): 14. doi:10.1186/s12896-016-0244-2. ISSN 1472-6750. PMC 4751646. PMID 26868129.
- "Identification and Experimental Characterization of an Extremophilic Brine Pool Alcohol Dehydrogenase from Single Amplified Genomes". dx.doi.org. doi:10.1021/acschembio.7b00792.s001. hdl:10754/626319. Retrieved 2023-03-16.
- Vogler, Malvina; Karan, Ram; Renn, Dominik; Vancea, Alexandra; Vielberg, Marie-Theres; Grötzinger, Stefan W.; DasSarma, Priya; DasSarma, Shiladitya; Eppinger, Jörg; Groll, Michael; Rueping, Magnus (2020-04-28). "Crystal Structure and Active Site Engineering of a Halophilic γ-Carbonic Anhydrase". Frontiers in Microbiology. 11: 742. doi:10.3389/fmicb.2020.00742. ISSN 1664-302X. PMC 7199487. PMID 32411108.
- Yoshimoto, Makoto; Walde, Peter (October 2018). "Immobilized carbonic anhydrase: preparation, characteristics and biotechnological applications". World Journal of Microbiology and Biotechnology. 34 (10): 151. doi:10.1007/s11274-018-2536-2. ISSN 0959-3993. PMID 30259182. S2CID 255141333.
- Ramadan, Eman; Maged, Mohamad; El Hosseiny, Ahmed; Chambergo, Felipe S.; Setubal, João C.; El Dorry, Hamza (2019-02-15). Master, Emma R. (ed.). "Molecular Adaptations of Bacterial Mercuric Reductase to the Hypersaline Kebrit Deep in the Red Sea". Applied and Environmental Microbiology. 85 (4): e01431–18. Bibcode:2019ApEnM..85E1431R. doi:10.1128/AEM.01431-18. ISSN 0099-2240. PMC 6365835. PMID 30504211.
- Batang, Zenon B.; Papathanassiou, Evangelos; Al-Suwailem, Abdulaziz; Smith, Chris; Salomidi, Maria; Petihakis, George; Alikunhi, Nabeel M.; Smith, Lloyd; Mallon, Francis; Yapici, Tahir; Fayad, Nabil (2012-06-01). "First discovery of a cold seep on the continental margin of the central Red Sea". Journal of Marine Systems. 94: 247–253. Bibcode:2012JMS....94..247B. doi:10.1016/j.jmarsys.2011.12.004. ISSN 0924-7963.
- Yang, Bo; Zhang, Weipeng; Tian, Renmao; Wang, Yong; Qian, Pei-Yuan (2015). "Changing composition of microbial communities indicates seepage fluid difference of the Thuwal Seeps in the Red Sea". Antonie van Leeuwenhoek. 108 (2): 461–471. doi:10.1007/s10482-015-0499-y. PMID 26059861. S2CID 254237594.