Arachnid
Arachnida (/əˈræknɪdə/) is a class of joint-legged invertebrate animals (arthropods), in the subphylum Chelicerata. Arachnida includes, among others, spiders, scorpions, ticks, mites, pseudoscorpions, harvestmen, camel spiders, whip spiders and vinegaroons.[1]
| Arachnids Temporal range: Early Silurian – present | |
|---|---|
 | |
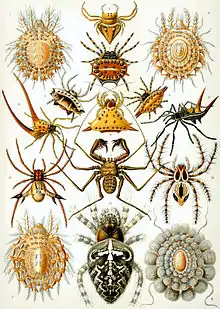
| Representatives of the 12 extant orders of arachnids | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Clade: | Bilateria |
| Clade: | Nephrozoa |
| (unranked): | Protostomia |
| Superphylum: | Ecdysozoa |
| (unranked): | Panarthropoda |
| (unranked): | Tactopoda |
| Phylum: | Arthropoda |
| Clade: | Arachnomorpha |
| Subphylum: | Chelicerata |
| Class: | Arachnida Lamarck, 1801 |
| Orders | |
| |
Adult arachnids have eight legs attached to the cephalothorax, although the frontmost pair of legs in some species has converted to a sensory function, while in other species, different appendages can grow large enough to take on the appearance of extra pairs of legs. The term is derived from the Greek word ἀράχνη (aráchnē, 'spider'), from the myth of the hubristic human weaver Arachne, who was turned into a spider.[2]
Almost all extant arachnids are terrestrial, living mainly on land. However, some inhabit freshwater environments and, with the exception of the pelagic zone, marine environments as well. They comprise over 110,000 named species, of which 51,000 are species of spiders.[3][4]
Morphology
Almost all adult arachnids have eight legs, unlike adult insects which all have six legs. However, arachnids also have two further pairs of appendages that have become adapted for feeding, defense, and sensory perception. The first pair, the chelicerae, serve in feeding and defense. The next pair of appendages, the pedipalps, have been adapted for feeding, locomotion, and/or reproductive functions. In scorpions, pseudoscorpions, and ricinuleids the pedipalps ends in a pair of pinchers, and in whip scorpions, Schizomida, Amblypygi, and most harvestmen, they are raptorial and used for prey capture.[5] In Solifugae, the palps are quite leg-like, so that these animals appear to have ten legs. The larvae of mites and Ricinulei have only six legs; a fourth pair usually appears when they moult into nymphs. However, mites are variable: as well as eight, there are adult mites with six or, like in Eriophyoidea, even four legs.[6][7] And while the adult males in some members of Podapolipidae have six legs, the adult females have only a single pair.[8]
Arachnids are further distinguished from insects by the fact they do not have antennae or wings. Their body is organized into two tagmata, called the prosoma, or cephalothorax, and the opisthosoma, or abdomen. (However, there is currently neither fossil nor embryological evidence that arachnids ever had a separate thorax-like division, so the validity of the term cephalothorax, which means a fused cephalon, or head, and thorax, has been questioned. There are also arguments against use of 'abdomen', as the opisthosoma of many arachnids contains organs atypical of an abdomen, such as a heart and respiratory organs.[9]) The prosoma, or cephalothorax, is usually covered by a single, unsegmented carapace. The abdomen is segmented in the more primitive forms, but varying degrees of fusion between the segments occur in many groups. It is typically divided into a preabdomen and postabdomen, although this is only clearly visible in scorpions, and in some orders, such as the Acari, the abdominal sections are completely fused.[10] A telson is present in scorpions, where it has been modified to a stinger, and into a flagellum in the Palpigradi, Schizomida (very short) and whip scorpions.[11] At the base of the flagellum in the two latter groups there are gland who produce acetic acid as a chemical defense.[12] Except for a pair of pectines in scorpions,[13] and the spinnerets in spiders, the abdomen has no appendages.[14]
Like all arthropods, arachnids have an exoskeleton, and they also have an internal structure of cartilage-like tissue, called the endosternite, to which certain muscle groups are attached. The endosternite is even calcified in some Opiliones.[15]
Locomotion
Most arachnids lack extensor muscles in the distal joints of their appendages. Spiders and whipscorpions extend their limbs hydraulically using the pressure of their hemolymph.[16] Solifuges and some harvestmen extend their knees by the use of highly elastic thickenings in the joint cuticle.[16] Scorpions, pseudoscorpions and some harvestmen have evolved muscles that extend two leg joints (the femur-patella and patella-tibia joints) at once.[17][18] The equivalent joints of the pedipalps of scorpions though, are extended by elastic recoil.[19]
Physiology
There are characteristics that are particularly important for the terrestrial lifestyle of arachnids, such as internal respiratory surfaces in the form of tracheae, or modification of the book gill into a book lung, an internal series of vascular lamellae used for gas exchange with the air.[20] While the tracheae are often individual systems of tubes, similar to those in insects, ricinuleids, pseudoscorpions, and some spiders possess sieve tracheae, in which several tubes arise in a bundle from a small chamber connected to the spiracle. This type of tracheal system has almost certainly evolved from the book lungs, and indicates that the tracheae of arachnids are not homologous with those of insects.[21]
Further adaptations to terrestrial life are appendages modified for more efficient locomotion on land, internal fertilisation, special sensory organs, and water conservation enhanced by efficient excretory structures as well as a waxy layer covering the cuticle.
The excretory glands of arachnids include up to four pairs of coxal glands along the side of the prosoma, and one or two pairs of Malpighian tubules, emptying into the gut. Many arachnids have only one or the other type of excretory gland, although several do have both. The primary nitrogenous waste product in arachnids is guanine.[21]
Arachnid blood is variable in composition, depending on the mode of respiration. Arachnids with an efficient tracheal system do not need to transport oxygen in the blood, and may have a reduced circulatory system. In scorpions and some spiders, however, the blood contains haemocyanin, a copper-based pigment with a similar function to haemoglobin in vertebrates. The heart is located in the forward part of the abdomen, and may or may not be segmented. Some mites have no heart at all.[21]
Diet and digestive system
Arachnids are mostly carnivorous, feeding on the pre-digested bodies of insects and other small animals. But ticks, and many mites, are parasites, some of which are carriers of disease. The diet of mites also include tiny animals, fungi, plant juices and decomposing matter.[22] Almost as varied is the diet of harvestmen, where we will find predators, decomposers and omnivores feeding on decaying plant and animal matter, droppings, animals and mushrooms.[23][24][25] The harvestmen and some mites, such as the house dust mite, are also the only arachnids able to ingest solid food, which exposes them to internal parasites,[26] although it is not unusual for spiders to eat their own silk. And one species of spider is mostly herbivorous.[27] Scorpions, spiders and pseudoscorpions secrete venom from specialized glands to kill prey or defend themselves.[28] Their venom also contains pre-digestive enzymes that helps breaking down the prey.[29][30][31] The saliva of ticks contains anticoagulants and anticomplements, and several species produce a neurotoxin.[32][33]
Arachnids produce digestive enzymes in their stomachs, and use their pedipalps and chelicerae to pour them over their dead prey. The digestive juices rapidly turn the prey into a broth of nutrients, which the arachnid sucks into a pre-buccal cavity located immediately in front of the mouth. Behind the mouth is a muscular, sclerotised pharynx, which acts as a pump, sucking the food through the mouth and on into the oesophagus and stomach. In some arachnids, the oesophagus also acts as an additional pump.
The stomach is tubular in shape, with multiple diverticula extending throughout the body. The stomach and its diverticula both produce digestive enzymes and absorb nutrients from the food. It extends through most of the body, and connects to a short sclerotised intestine and anus in the hind part of the abdomen.[21]
Senses
Arachnids have two kinds of eyes: the lateral and median ocelli. The lateral ocelli evolved from compound eyes and may have a tapetum, which enhances the ability to collect light. With the exception of scorpions, which can have up to five pairs of lateral ocelli, there are never more than three pairs present. The median ocelli develop from a transverse fold of the ectoderm. The ancestors of modern arachnids probably had both types, but modern ones often lack one type or the other.[26] The cornea of the eye also acts as a lens, and is continuous with the cuticle of the body. Beneath this is a transparent vitreous body, and then the retina and, if present, the tapetum. In most arachnids, the retina probably does not have enough light sensitive cells to allow the eyes to form a proper image.[21]
In addition to the eyes, almost all arachnids have two other types of sensory organs. The most important to most arachnids are the fine sensory hairs that cover the body and give the animal its sense of touch. These can be relatively simple, but many arachnids also possess more complex structures, called trichobothria.
Finally, slit sense organs are slit-like pits covered with a thin membrane. Inside the pit, a small hair touches the underside of the membrane, and detects its motion. Slit sense organs are believed to be involved in proprioception, and possibly also hearing.[21]
Reproduction
_(8390306848).jpg.webp)
Arachnids may have one or two gonads, which are located in the abdomen. The genital opening is usually located on the underside of the second abdominal segment. In most species, the male transfers sperm to the female in a package, or spermatophore. The males in harvestmen and some mites have a penis.[34] Complex courtship rituals have evolved in many arachnids to ensure the safe delivery of the sperm to the female.[21] Members of many orders exhibit sexual dimorphism.[35]
Arachnids usually lay yolky eggs, which hatch into immatures that resemble adults. Scorpions, however, are either ovoviviparous or viviparous, depending on species, and bear live young. Also some mites are ovoviviparous and viviparous, even if most lay eggs.[36] In most arachnids only the females provide parental care, with harvestmen being one of the few exceptions.[37][38]
Taxonomy and evolution
Phylogeny
The phylogenetic relationships among the main subdivisions of arthropods have been the subject of considerable research and dispute for many years. A consensus emerged from about 2010 onwards, based on both morphological and molecular evidence. Extant (living) arthropods are a monophyletic group and are divided into three main clades: chelicerates (including arachnids), pancrustaceans (the paraphyletic crustaceans plus insects and their allies), and myriapods (centipedes, millipedes and allies).[39][40][41][42][43] The three groups are related as shown in the cladogram below.[41] Including fossil taxa does not fundamentally alter this view, although it introduces some additional basal groups.[44]
| Arthropoda |
| ||||||||||||
The extant chelicerates comprise two marine groups: sea spiders and horseshoe crabs, and the terrestrial arachnids. These have been thought to be related as shown below.[40][43] (Pycnogonida (sea spiders) may be excluded from the chelicerates, which are then identified as the group labelled "Euchelicerata".[45]) A 2019 analysis nests Xiphosura deeply within Arachnida.[46]
| Chelicerata |
| ||||||||||||
Discovering relationships within the arachnids has proven difficult as of March 2016, with successive studies producing different results. A study in 2014, based on the largest set of molecular data to date, concluded that there were systematic conflicts in the phylogenetic information, particularly affecting the orders Acariformes, Parasitiformes and Pseudoscorpiones, which have had much faster evolutionary rates. Analyses of the data using sets of genes with different evolutionary rates produced mutually incompatible phylogenetic trees. The authors favoured relationships shown by more slowly evolving genes, which demonstrated the monophyly of Chelicerata, Euchelicerata and Arachnida, as well as of some clades within the arachnids. The diagram below summarizes their conclusions, based largely on the 200 most slowly evolving genes; dashed lines represent uncertain placements.[43]
|
Arachnopulmonata |

Tetrapulmonata, here consisting of Araneae, Amblypygi and Uropygi (Thelyphonida s.s.) (Schizomida was not included in the study), received strong support. Somewhat unexpectedly, there was support for a clade comprising Opiliones, Ricinulei and Solifugae, a combination not found in most other studies.[43] In early 2019, a molecular phylogenetic analysis placed the horseshoe crabs, Xiphosura, as the sister group to Ricinulei. It also grouped pseudoscorpions with mites and ticks, which the authors considered may be due to long branch attraction.[46] The addition of Scorpiones to produce a clade called Arachnopulmonata was also well supported. Pseudoscorpiones may also belong here, as all six orders share the same ancient whole genome duplication,[47] and analyses support pseudoscorpions as the sister group of scorpions.[48] Genetic analysis has not yet been done for Ricinulei, Palpigradi, or Solifugae, but horseshoe crabs have gone through two whole genome duplications, which gives them five Hox clusters with 34 Hox genes, the highest number found in any invertebrate, yet it is not clear if the oldest genome duplication is related to the one in Arachnopulmonata.[49][50]
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
More recent phylogenomic analyses that have densely sampled both genomic datasets and morphology have consistently supported horseshoe crabs as nested inside Arachnida, suggesting a complex history of terrestrialization.[51][52] Morphological analyses including fossils tend to recover the Tetrapulmonata, including the extinct group the Haptopoda,[53][54][55][56][57] but recover other ordinal relationships with low support.
Fossil history


The Uraraneida are an extinct order of spider-like arachnids from the Devonian and Permian.[58]
A fossil arachnid in 100 million year old (mya) amber from Myanmar, Chimerarachne yingi, has spinnerets (to produce silk); it also has a tail, like the Palaeozoic Uraraneida, some 200 million years after other known fossils with tails. The fossil resembles the most primitive living spiders, the mesotheles.[59][53]
Taxonomy

The subdivisions of the arachnids are usually treated as orders. Historically, mites and ticks were treated as a single order, Acari. However, molecular phylogenetic studies suggest that the two groups do not form a single clade, with morphological similarities being due to convergence. They are now usually treated as two separate taxa – Acariformes, mites, and Parasitiformes, ticks – which may be ranked as orders or superorders. The arachnid subdivisions are listed below alphabetically; numbers of species are approximate.
- Extant forms
- Acariformes – mites (32,000 species)
- Amblypygi – "blunt rump" tail-less whip scorpions with front legs modified into whip-like sensory structures as long as 25 cm or more (250 species)
- Araneae – spiders (51,000 species)
- Opiliones – phalangids, harvestmen or daddy-long-legs (6,700 species)
- Palpigradi – microwhip scorpions (130 species)
- Parasitiformes – ticks (12,000 species)
- Pseudoscorpionida – pseudoscorpions (4,000 species)
- Ricinulei – ricinuleids, hooded tickspiders (100 species)
- Schizomida – "split middle" whip scorpions with divided exoskeletons (350 species)
- Scorpiones – scorpions (2,700 species)
- Solifugae – solpugids, windscorpions, sun spiders or camel spiders (1,200 species)
- Uropygi (also called Thelyphonida) – whip scorpions or vinegaroons, forelegs modified into sensory appendages and a long tail on abdomen tip (120 species)
- Extinct forms
- †Haptopoda – extinct arachnids apparently part of the Tetrapulmonata, the group including spiders and whip scorpions (1 species)
- †Phalangiotarbida – extinct arachnids of uncertain affinity (30 species)
- †Trigonotarbida – extinct (late Silurian Early Permian)
- †Uraraneida – extinct spider-like arachnids, but with a "tail" and no spinnerets (2 species)
It is estimated that 110,000 arachnid species have been described, and that there may be over a million in total.[4]
References
- Cracraft, Joel & Donoghue, Michael, eds. (2004). Assembling the Tree of Life. Oxford University Press. p. 297.
- "Arachnid". Oxford English Dictionary (2nd ed.). 1989.
- Brabazon, Anthony (2018). Foraging-Inspired Optimisation Algorithms. Springer International Publishing. p. 237. ISBN 9783319591568.
- Agnarsson, Ingi (2023). "Grand challenges in research on arachnid diversity, conservation, and biogeography". Frontiers in Arachnid Science. 2. doi:10.3389/frchs.2023.1101141.
- Schierwater, Bernd; DeSalle, Rob (2021-07-08). Invertebrate Zoology: A Tree of Life Approach. CRC Press. ISBN 978-1-4822-3582-1.
- Schmidt, Günther (1993). Giftige und gefährliche Spinnentiere [Poisonous and dangerous arachnids] (in German). Westarp Wissenschaften. p. 75. ISBN 978-3-89432-405-6.
- Bolton, Samuel J.; Chetverikov, Philipp E.; Klompen, Hans (2017). "Morphological support for a clade comprising two vermiform mite lineages: Eriophyoidea (Acariformes) and Nematalycidae (Acariformes)". Systematic and Applied Acarology. 22 (8): 1096. doi:10.11158/saa.22.8.2. S2CID 90899467.
- Dhooria, Manjit Singh (2016-12-14). Fundamentals of Applied Acarology. Springer. ISBN 978-981-10-1594-6.
- Shultz, Stanley; Shultz, Marguerite (2009). The Tarantula Keeper's Guide. Hauppauge, New York: Barron's. p. 23. ISBN 978-0-7641-3885-0.
- Ruppert, E.; Fox, R. & Barnes, R. (2007). Invertebrate Zoology: A Functional Evolutionary Approach (7th ed.). Thomson Learning. ISBN 978-0-03-025982-1.
- Little, Colin (1983-12-15). The Colonisation of Land: Origins and Adaptations of Terrestrial Animals. Cambridge University Press. ISBN 978-0-521-25218-8.
- Pinto-da-Rocha, Ricardo; Machado, Glauco; Giribet, Gonzalo (2007-02-28). Harvestmen: The Biology of Opiliones. Harvard University Press. ISBN 978-0-674-02343-7.
- Di, Z.; Edgecombe, G. D.; Sharma, P. P. (2018). "Homeosis in a scorpion supports a telopodal origin of pectines and components of the book lungs". BMC Evolutionary Biology. 18 (1): 73. doi:10.1186/s12862-018-1188-z. PMC 5963125. PMID 29783957.
- Selden, Paul; Shear, William (2008). "Fossil evidence for the origin of spider spinnerets". Nature Precedings: 1. doi:10.1038/npre.2008.2088.1.
- Kovoor, J. (1978). "Natural calcification of the prosomatic endosternite in the Phalangiidae (Arachnida:Opiliones)". Calcified Tissue Research. 26 (3): 267–269. doi:10.1007/BF02013269. PMID 750069. S2CID 23119386.
- Sensenig, Andrew T. & Shultz, Jeffrey W. (February 15, 2003). "Mechanics of Cuticular Elastic Energy Storage in Leg Joints Lacking Extensor Muscles in Arachnids". Journal of Experimental Biology. 206 (4): 771–784. doi:10.1242/jeb.00182. ISSN 1477-9145. PMID 12517993. S2CID 40503319.
- Shultz, Jeffrey W. (February 6, 2005). "Evolution of locomotion in arachnida: The hydraulic pressure pump of the giant whipscorpion, Mastigoproctus giganteus (Uropygi)". Journal of Morphology. 210 (1): 13–31. doi:10.1002/jmor.1052100103. ISSN 1097-4687. PMID 29865543. S2CID 46935000.
- Shultz, Jeffrey W. (January 1, 1992). "Muscle Firing Patterns in Two Arachnids Using Different Methods of Propulsive Leg Extension". Journal of Experimental Biology. 162 (1): 313–329. doi:10.1242/jeb.162.1.313. ISSN 1477-9145. Retrieved 2012-05-19.
- Sensenig, Andrew T. & Shultz, Jeffrey W. (2004). "Elastic energy storage in the pedipedal joints of scorpions and sun-spiders (Arachnida, Scorpiones, Solifugae)". Journal of Arachnology. 32 (1): 1–10. doi:10.1636/S02-73. ISSN 0161-8202. S2CID 56461501.
- Garwood, Russell J. & Edgecombe, Gregory D. (September 2011). "Early Terrestrial Animals, Evolution, and Uncertainty". Evolution: Education and Outreach. 4 (3): 489–501. doi:10.1007/s12052-011-0357-y.
- Barnes, Robert D. (1982). Invertebrate Zoology. Philadelphia, PA: Holt-Saunders International. pp. 596–604. ISBN 978-0-03-056747-6.
- Potapov, Anton M.; Beaulieu, Frédéric; Birkhofer, Klaus; Bluhm, Sarah L.; Degtyarev, Maxim I.; Devetter, Miloslav; Goncharov, Anton A.; Gongalsky, Konstantin B.; Klarner, Bernhard; Korobushkin, Daniil I.; Liebke, Dana F.; Maraun, Mark; Mc Donnell, Rory J.; Pollierer, Melanie M.; Schaefer, Ina; Shrubovych, Julia; Semenyuk, Irina I.; Sendra, Alberto; Tuma, Jiri; Tůmová, Michala; Vassilieva, Anna B.; Chen, Ting‐Wen; Geisen, Stefan; Schmidt, Olaf; Tiunov, Alexei V.; Scheu, Stefan (2022). "Feeding habits and multifunctional classification of soil‐associated consumers from protists to vertebrates". Biological Reviews. 97 (3): 1057–1117. doi:10.1111/brv.12832. PMID 35060265. S2CID 246078291.
- Powell, Erin C.; Painting, Christina J.; Hickey, Anthony J.; Machado, Glauco; Holwell, Gregory I. (2021). "Diet, predators, and defensive behaviors of New Zealand harvestmen (Opiliones: Neopilionidae)". The Journal of Arachnology. 49. doi:10.1636/JoA-S-20-002. S2CID 234364795.
- Common harvestman | The Wildlife Trusts
- How do harvestmen hunt? - BBC Wildlife Magazine
- Machado, Glauco; Pinto-da-Rocha, Ricardo & Giribet, Gonzalo (2007). Pinto-da-Rocha, Ricardo; Machado, Glauco & Giribet, Gonzalo (eds.). Harvestmen: the Biology of Opiliones. Harvard University Press. ISBN 978-0-674-02343-7.
- Rare Vegetarian Spider Discovered
- Santibáñez-López, C. E.; Ontano, A. Z.; Harvey, M. S.; Sharma, P. P. (2018). "Transcriptomic Analysis of Pseudoscorpion Venom Reveals a Unique Cocktail Dominated by Enzymes and Protease Inhibitors". Toxins. 10 (5): 207. doi:10.3390/toxins10050207. PMC 5983263. PMID 29783636.
- Zeh, J. A.; Bonilla, M. M.; Adrian, A. J.; Mesfin, S.; Zeh, D. W. (2012). "From father to son: Transgenerational effect of tetracycline on sperm viability". Scientific Reports. 2: 375. Bibcode:2012NatSR...2E.375Z. doi:10.1038/srep00375. PMC 3337657. PMID 22540028.
- Delgado-Prudencio, G.; Cid-Uribe, J. I.; Morales, J. A.; Possani, L. D.; Ortiz, E.; Romero-Gutiérrez, T. (2022). "The Enzymatic Core of Scorpion Venoms". Toxins. 14 (4): 248. doi:10.3390/toxins14040248. PMC 9030722. PMID 35448857.
- Walter, A.; Bechsgaard, J.; Scavenius, C.; Dyrlund, T. S.; Sanggaard, K. W.; Enghild, J. J.; Bilde, T. (2017). "Characterisation of protein families in spider digestive fluids and their role in extra-oral digestion". BMC Genomics. 18 (1): 600. doi:10.1186/s12864-017-3987-9. PMC 5553785. PMID 28797246.
- Denisov, S. S.; Ippel, J. H.; Castoldi, E.; Mans, B. J.; Hackeng, T. M.; Dijkgraaf, I. (2021). "Molecular basis of anticoagulant and anticomplement activity of the tick salivary protein Salp14 and its homologs". The Journal of Biological Chemistry. 297 (1): 100865. doi:10.1016/j.jbc.2021.100865. PMC 8294578. PMID 34118237.
- Tick Paralysis - StatPearls - NCBI Bookshelf
- McLean, C. J.; Garwood, R. J.; Brassey, C. A. (2018). "Sexual dimorphism in the Arachnid orders". PeerJ. 6: e5751. doi:10.7717/peerj.5751. PMC 6225839. PMID 30416880.
- McLean, Callum J.; Garwood, Russell J.; Brassey, Charlotte A. (2018). "Sexual dimorphism in the Arachnid orders". PeerJ. 6: e5751. doi:10.7717/peerj.5751. ISSN 2167-8359. PMC 6225839. PMID 30416880.
- Auerbach, Paul S. (2011-10-31). Wilderness Medicine E-Book: Expert Consult Premium Edition - Enhanced Online Features. Elsevier Health Sciences. ISBN 978-1-4557-3356-9.
- Quesada-Hidalgo, Rosannette; Solano-Brenes, Diego; Requena, Gustavo S.; Machado, Glauco (April 2019). "The good fathers: efficiency of male care and the protective role of foster parents in a Neotropical arachnid". Animal Behaviour. 150: 147–155. doi:10.1016/j.anbehav.2019.02.007. S2CID 73728615.
- Nazareth, Tais M.; Machado, Glauco (March 2010). "Mating system and exclusive postzygotic paternal care in a Neotropical harvestman (Arachnida: Opiliones)". Animal Behaviour. 79 (3): 547–554. doi:10.1016/j.anbehav.2009.11.026. S2CID 53166528.
- Meusemann, Karen; Reumont, Björn M. von; Simon, Sabrina; Roeding, Falko; Strauss, Sascha; Kück, Patrick; Ebersberger, Ingo; Walzl, Manfred; Pass, Günther; Breuers, Sebastian; Achter, Viktor; Haeseler, Arndt von; Burmester, Thorsten; Hadrys, Heike; Wägele, J. Wolfgang & Misof, Bernhard (2010). "A Phylogenomic Approach to Resolve the Arthropod Tree of Life". Molecular Biology and Evolution. 27 (11): 2451–2464. doi:10.1093/molbev/msq130. PMID 20534705.
- Regier, Jerome C.; Shultz, Jeffrey W.; Zwick, Andreas; Hussey, April; Ball, Bernard; Wetzer, Regina; Martin, Joel W. & Cunningham, Clifford W. (2010). "Arthropod relationships revealed by phylogenomic analysis of nuclear protein-coding sequences". Nature. 463 (7284): 1079–1083. Bibcode:2010Natur.463.1079R. doi:10.1038/nature08742. PMID 20147900. S2CID 4427443.
- Rota-Stabelli, Omar; Campbell, Lahcen; Brinkmann, Henner; Edgecombe, Gregory D.; Longhorn, Stuart J.; Peterson, Kevin J.; Pisani, Davide; Philippe, Hervé & Telford, Maximilian J. (2010). "A congruent solution to arthropod phylogeny: phylogenomics, microRNAs and morphology support monophyletic Mandibulata". Proceedings of the Royal Society of London B: Biological Sciences. 278 (1703): 298–306. doi:10.1098/rspb.2010.0590. PMC 3013382. PMID 20702459.
- Campbell, Lahcen I.; Rota-Stabelli, Omar; Edgecombe, Gregory D.; Marchioro, Trevor; Longhorn, Stuart J.; Telford, Maximilian J.; Philippe, Hervé; Rebecchi, Lorena; Peterson, Kevin J. & Pisani, Davide (2011). "MicroRNAs and phylogenomics resolve the relationships of Tardigrada and suggest that velvet worms are the sister group of Arthropoda". Proceedings of the National Academy of Sciences. 108 (38): 15920–15924. Bibcode:2011PNAS..10815920C. doi:10.1073/pnas.1105499108. PMC 3179045. PMID 21896763.
- Sharma, Prashant P.; Kaluziak, Stefan T.; Pérez-Porro, Alicia R.; González, Vanessa L.; Hormiga, Gustavo; Wheeler, Ward C. & Giribet, Gonzalo (2014-01-11). "Phylogenomic Interrogation of Arachnida Reveals Systemic Conflicts in Phylogenetic Signal". Molecular Biology and Evolution. 31 (11): 2963–2984. doi:10.1093/molbev/msu235. PMID 25107551.
- Legg, David A.; Sutton, Mark D. & Edgecombe, Gregory D. (2013). "Arthropod fossil data increase congruence of morphological and molecular phylogenies". Nature Communications. 4: 2485. Bibcode:2013NatCo...4.2485L. doi:10.1038/ncomms3485. PMID 24077329.
- Giribet, Gonzalo; Edgecombe, Gregory D. & Wheeler, Ward C. (2001). "Arthropod phylogeny based on eight molecular loci and morphology". Nature. 413 (6852): 157–161. Bibcode:2001Natur.413..157G. doi:10.1038/35093097. PMID 11557979. S2CID 4431635.
- Ballesteros, J. A.; Sharma, P. P. (2019). "A Critical Appraisal of the Placement of Xiphosura (Chelicerata) with Account of Known Sources of Phylogenetic Error". Systematic Biology. 68 (6): 896–917. doi:10.1093/sysbio/syz011. PMID 30917194.
- Gainett, Guilherme; González, Vanessa L.; Ballesteros, Jesús A.; Setton, Emily V. W.; Baker, Caitlin M.; Gargiulo, Leonardo Barolo; Santibáñez-López, Carlos E.; Coddington, Jonathan A.; Sharma, Prashant P. (2021). "The genome of a daddy-long-legs (Opiliones) illuminates the evolution of arachnid appendages and chelicerate genome architecture" (PDF). doi:10.1101/2021.01.11.426205. S2CID 231614575.
{{cite journal}}: Cite journal requires|journal=(help) - Garbiec, Arnold; Christophoryová, Jana; Jędrzejowska, Izabela (2022). "Spectacular alterations in the female reproductive system during the ovarian cycle and adaptations for matrotrophy in chernetid pseudoscorpions (Pseudoscorpiones: Chernetidae)". Scientific Reports. 12 (1): 6447. Bibcode:2022NatSR..12.6447G. doi:10.1038/s41598-022-10283-z. PMC 9018881. PMID 35440674.
- Taxonomic Sampling and Rare Genomic Changes Overcome Long-Branch Attraction in the Phylogenetic Placement of Pseudoscorpions
- Chromosome-level assembly of the horseshoe crab genome provides insights into its genome evolution
- Ballesteros, Jesús A.; Santibáñez López, Carlos E.; Kováč, Ľubomír; Gavish-Regev, Efrat; Sharma, Prashant P. (2019-12-18). "Ordered phylogenomic subsampling enables diagnosis of systematic errors in the placement of the enigmatic arachnid order Palpigradi". Proceedings of the Royal Society B: Biological Sciences. 286 (1917): 20192426. doi:10.1098/rspb.2019.2426. ISSN 0962-8452. PMC 6939912. PMID 31847768.
- Ballesteros, Jesús A; Santibáñez-López, Carlos E; Baker, Caitlin M; Benavides, Ligia R; Cunha, Tauana J; Gainett, Guilherme; Ontano, Andrew Z; Setton, Emily V W; Arango, Claudia P; Gavish-Regev, Efrat; Harvey, Mark S; Wheeler, Ward C; Hormiga, Gustavo; Giribet, Gonzalo; Sharma, Prashant P (2022-02-03). Teeling, Emma (ed.). "Comprehensive Species Sampling and Sophisticated Algorithmic Approaches Refute the Monophyly of Arachnida". Molecular Biology and Evolution. 39 (2): msac021. doi:10.1093/molbev/msac021. ISSN 0737-4038. PMC 8845124. PMID 35137183.
- Wang, B.; Dunlop, J.A.; Selden, P.A.; Garwood, R.J.; Shear, W.A.; Müller, P.; Lei, X. (2018). "Cretaceous arachnid Chimerarachne yingi gen. et sp. nov. illuminates spider origins". Nature Ecology & Evolution. 2 (4): 614–622. doi:10.1038/s41559-017-0449-3. PMID 29403075. S2CID 4239867.
- Garwood, R.J.; Dunlop, J.A.; Knecht, B.J.; Hegna, T.A. (2017). "The phylogeny of fossil whip spiders". BMC Evolutionary Biology. 17 (1): 105. doi:10.1186/s12862-017-0931-1. PMC 5399839. PMID 28431496.
- Garwood, R.J.; Dunlop, J.A.; Selden, P.A.; Spencer, A.R.T.; Atwood, R.C.; Vo, N.T.; Drakopoulos, M. (2016). "Almost a spider: a 305-million-year-old fossil arachnid and spider origins". Proceedings of the Royal Society B: Biological Sciences. 283 (1827): 20160125. doi:10.1098/rspb.2016.0125. PMC 4822468. PMID 27030415.
- Garwood, R.J.; Dunlop, J. (2014). "Three-dimensional reconstruction and the phylogeny of extinct chelicerate orders". PeerJ. 2: e641. doi:10.7717/peerj.641. PMC 4232842. PMID 25405073.
- Shultz, J.W. (2007). "A phylogenetic analysis of the arachnid orders based on morphological characters". Zoological Journal of the Linnean Society. 150 (2): 221–265. doi:10.1111/j.1096-3642.2007.00284.x.
- Selden, P.A.; Shear, W.A. & Sutton, M.D. (2008), "Fossil evidence for the origin of spider spinnerets, and a proposed arachnid order", Proceedings of the National Academy of Sciences, 105 (52): 20781–20785, Bibcode:2008PNAS..10520781S, doi:10.1073/pnas.0809174106, PMC 2634869, PMID 19104044
- Briggs, Helen (5 February 2018). "'Extraordinary' fossil sheds light on origins of spiders". BBC. Retrieved 9 June 2018.

.jpg.webp)
_(white_background).jpg.webp)
.png.webp)
.png.webp)

_Figure_1.jpg.webp)


.png.webp)
.png.webp)


