Sirtuin 3
NAD-dependent deacetylase sirtuin-3, mitochondrial also known as SIRT3 is a protein that in humans is encoded by the SIRT3 gene [sirtuin (silent mating type information regulation 2 homolog) 3 (S. cerevisiae)].[5][6] SIRT3 is member of the mammalian sirtuin family of proteins, which are homologs to the yeast Sir2 protein. SIRT3 exhibits NAD+-dependent deacetylase activity.
Members of the sirtuin family are characterized by a sirtuin core domain and grouped into four classes, and the protein encoded by this gene is included in class I of the sirtuin family.[5] The human sirtuins have a range of molecular functions and have emerged as important proteins in aging, stress resistance, and metabolic regulation. Yeast sirtuin proteins are known to regulate epigenetic gene silencing and suppress recombination of rDNA.[7] In addition to protein deacetylation, studies have shown that the human sirtuins may also function as intracellular regulatory proteins with mono ADP ribosyltransferase activity.
Structure
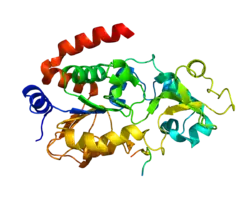
SIRT3 is a soluble protein located in the mitochondrial matrix, and contains a mitochondrial processing peptide at the N-terminus. A set of crystal structures of human SIRT3 have been solved, including an apo-structure with no substrate, a structure with a peptide containing acetyl lysine of its natural substrate acetyl-CoA synthetase 2, a reaction intermediate structure trapped by a thioacetyl peptide, and a structure with the dethioacetylated peptide bond.[8] These structures show the conformational changes induced by the two substrates required for the reaction, the acetylated substrate peptide and NAD+. In addition, a binding study by isothermal titration calorimetry suggests that the acetylated peptide is the first substrate to bind to SIRT3, prior to NAD+.
Function
Mitochondrial
Three sirtuins, SIRT3, SIRT4 and SIRT5, are located in mitochondria and have been implicated in regulating metabolic processes. Endogenous SIRT3 is a soluble protein located in the mitochondrial matrix.[9] Overexpression of SIRT3 in cultured cells increases respiration and decreases the production of reactive oxygen species. Fasting increases SIRT3 expression in white and brown adipose tissue (WAT and BAT, respectively) and overexpression of SIRT3 in HIB1B brown adipocytes increases the expression of PGC-1α and UCP1, suggesting a role for SIRT3 in adaptive thermogenesis BAT. BAT is different from WAT because it harbors large numbers of mitochondria and is important for thermogenesis in rodents. Thermogenesis in BAT is mediated by the uncoupling protein 1 (UCP1), which induces proton leakage and thereby generates heat instead of ATP. Mechanistic insights into how SIRT3 affects thermogenesis in BAT is lacking and whether SIRT3 affects UCP1 activity directly is not known.
In addition to controlling metabolism at the transcriptional level, sirtuins also directly control the activity of metabolic enzymes. In Salmonella enterica, the bacterial sirtuin CobB regulates the activity of the enzyme acetyl-coenzyme A (acetyl-CoA) synthetase. As mentioned above, orthologs of acetyl-CoA synthetase exist in the cytoplasm (AceCS1) and in mitochondria (AceCS2) in mammals. The presence of the sirtuin deacetylase SIRT3 in the mitochondrial matrix suggests the existence of lysine acetylated mitochondrial proteins. Indeed, SIRT3 deacetylates and activates the mammalian mitochondrial acetyl-coA synthetase (AceCS2). Furthermore, SIRT3 and AceCS2 are found complexed with one another, suggesting a critical role for control of AceCS2 activity by SIRT3.
Activation of the NMNAT2 enzyme, which catalyze an essential step in the nicotinamide adenine dinucleotide (NAD+) biosynthetic pathway by SIRT3 may be a means of inhibiting axon degeneration and dysfunction.[10]
Nuclear
In addition to its reported mitochondrial function, some researchers have proposed a very small pool of active nuclear SIRT3 exists. This pool is reported to consist of the long form of SIRT3 and has been suggested to have histone deacetylase activity.[11] The observation that SIRT3 has nuclear activity came from a report that SIRT3 protected cardiomyocytes from stress mediated cell death and that this effect was due to deacetylation of a nuclear factor, Ku-70.[12]
Clinical significance
Aging
There is a strong association between SIRT3 alleles and longevity in males.[13]
Activation of SIRT3 inhibits the apoptosis leading to age-related macular degeneration.[10] SIRT3 induced mitophagy, inhibiting cell death, and thus could be used to treat neurodegenerative diseases.[10]
Carcinogenesis
There is a significant body of published literature suggesting a strong mechanistic link between mitochondrial function, aging, and carcinogenesis.[13] SIRT3 inhibits cancers that depend upon glycolysis, but promotes cancers that depend upon oxidative phosphorylation.[10]
Sirt3 functions as a mitochondrial tumor suppressor protein. Although some evidence attributes SIRT3 activity in bypassing growth arrest in bladder carcinoma cells via regulation of p53 in the mitochondria.[14] Damaged and aberrant mitochondrial function, similar to gene mutations, may be an early event that ultimately leads to the development of cancers. Mice genetically altered to delete Sirt3 develop estrogen and progesterone receptor (ER/PR) positive breast mammary tumors. In tumor samples from women with breast cancer, SIRT3 expression was decreased, as compared to normal breast tissues. Thus, the Sirt3 knockout model may be used to investigate ER/PR positive breast tumor development.[15]
References
- GRCh38: Ensembl release 89: ENSG00000142082 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000025486 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Entrez Gene: SIRT3 sirtuin (silent mating type information regulation 2 homolog) 3 (S. cerevisiae)".
- Schwer B, North BJ, Frye RA, Ott M, Verdin E (August 2002). "The human silent information regulator (Sir)2 homologue hSIRT3 is a mitochondrial nicotinamide adenine dinucleotide-dependent deacetylase". Journal of Cell Biology. 158 (4): 647–57. doi:10.1083/jcb.200205057. PMC 2174009. PMID 12186850.
- Gartenberg MR, Smith JS (August 2016). "The Nuts and Bolts of Transcriptionally Silent Chromatin in Saccharomyces cerevisiae". Genetics. 203 (4): 1563–1599. doi:10.1534/genetics.112.145243. PMC 4981263. PMID 27516616.
- PDB: 3GLR, 3GLS, 3GLT, 3GLU; Jin L, Wei W, Jiang Y, Peng H, Cai J, Mao C, Dai H, Choy W, Bemis JE, Jirousek MR, Milne JC, Westphal CH, Perni RB (September 2009). "Crystal structures of human SIRT3 displaying substrate-induced conformational changes". J. Biol. Chem. 284 (36): 24394–405. doi:10.1074/jbc.M109.014928. PMC 2782032. PMID 19535340.
- Onyango P, Celic I, McCaffery JM, Boeke JD, Feinberg AP (October 2002). "SIRT3, a human SIR2 homologue, is an NAD-dependent deacetylase localized to mitochondria". Proceedings of the National Academy of Sciences of the United States of America. 99 (21): 13653–58. Bibcode:2002PNAS...9913653O. doi:10.1073/pnas.222538099. PMC 129731. PMID 12374852.
- Zhang J, Xiang H, Rong-Rong He R, Liu B (2020). "Mitochondrial Sirtuin 3: New emerging biological function and therapeutic target". Theranostics. 10 (18): 8315–8342. doi:10.7150/thno.45922. PMC 7381741. PMID 32724473.
- Scher MB, Vaquero A, Reinberg D (April 2007). "SirT3 is a nuclear NAD+-dependent histone deacetylase that translocates to the mitochondria upon cellular stress". Genes Dev. 21 (8): 920–28. doi:10.1101/gad.1527307. PMC 1847710. PMID 17437997.
- Sundaresan NR, Samant SA, Pillai VB, Rajamohan SB, Gupta MP (August 2008). "SIRT3 is a stress responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku-70". Mol. Cell. Biol. 28 (20): 6384–401. doi:10.1128/MCB.00426-08. PMC 2577434. PMID 18710944.
- Bellizzi D, Rose G, Cavalcante P, Covello G, Dato S, De Rango F, Greco V, Maggiolini M, Feraco E, Mari V, Franceschi C, Passarino G, De Benedictis G (February 2005). "A novel VNTR enhancer within the SIRT3 gene, a human homologue of SIR2, is associated with survival at oldest ages". Genomics. 85 (2): 258–63. doi:10.1016/j.ygeno.2004.11.003. PMID 15676284.
- Li S, Banck M, Mujtaba S, Zhou MM, Sugrue MM, Walsh MJ (2010). "p53-Induced growth arrest Is regulated by the mitochondrial SirT3 deacetylase". PLOS ONE. 5 (5): e10486. Bibcode:2010PLoSO...510486L. doi:10.1371/journal.pone.0010486. PMC 2864751. PMID 20463968.
- Kim HS, Patel K, Muldoon-Jacobs K, Bisht KS, Aykin-Burns N, Pennington JD, van der Meer R, Nguyen P, Savage J, Owens KM, Vassilopoulos A, Ozden O, Park SH, Singh KK, Abdulkadir SA, Spitz DR, Deng CX, Gius D (January 2010). "SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress". Cancer Cell. 17 (1): 41–52. doi:10.1016/j.ccr.2009.11.023. PMC 3711519. PMID 20129246.
Further reading
- Bellizzi D, Dato S, Cavalcante P, Covello G, Di Cianni F, Passarino G, Rose G, De Benedictis G (2007). "Characterization of a bidirectional promoter shared between two human genes related to aging: SIRT3 and PSMD13". Genomics. 89 (1): 143–50. doi:10.1016/j.ygeno.2006.09.004. PMID 17059877.
- Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, Li N, Berriz GF, Gibbons FD, Dreze M, Ayivi-Guedehoussou N, Klitgord N, Simon C, Boxem M, Milstein S, Rosenberg J, Goldberg DS, Zhang LV, Wong SL, Franklin G, Li S, Albala JS, Lim J, Fraughton C, Llamosas E, Cevik S, Bex C, Lamesch P, Sikorski RS, Vandenhaute J, Zoghbi HY, Smolyar A, Bosak S, Sequerra R, Doucette-Stamm L, Cusick ME, Hill DE, Roth FP, Vidal M (2005). "Towards a proteome-scale map of the human protein-protein interaction network". Nature. 437 (7062): 1173–78. Bibcode:2005Natur.437.1173R. doi:10.1038/nature04209. PMID 16189514. S2CID 4427026.
- Yang YH, Chen YH, Zhang CY, Nimmakayalu MA, Ward DC, Weissman S (2001). "Cloning and characterization of two mouse genes with homology to the yeast Sir2 gene". Genomics. 69 (3): 355–69. doi:10.1006/geno.2000.6360. PMID 11056054.
- Frye RA (2000). "Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins". Biochem. Biophys. Res. Commun. 273 (2): 793–98. doi:10.1006/bbrc.2000.3000. PMID 10873683.
- Frye RA (1999). "Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity". Biochem. Biophys. Res. Commun. 260 (1): 273–79. doi:10.1006/bbrc.1999.0897. PMID 10381378.
- Bonaldo MF, Lennon G, Soares MB (1997). "Normalization and subtraction: two approaches to facilitate gene discovery". Genome Res. 6 (9): 791–806. doi:10.1101/gr.6.9.791. PMID 8889548.