SorCS2
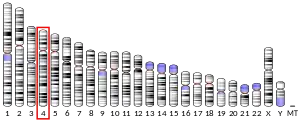
The SorCS2 (sortilin-related Vps10p domain containing receptor 2) gene is found on chromosome 4 (4p16.1), and is composed of 28 exons. The N-terminal exons which encode the Vps10p domain are spaced by large introns. The functional receptor protein is largely present in the brain. It is 1109 amino acids long, largely neutral, and has a single transmembrane pass....[5]
| SORCS2 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | SORCS2, sortilin related VPS10 domain containing receptor 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 606284 MGI: 1932289 HomoloGene: 56899 GeneCards: SORCS2 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
SorCS2 is a member of the mammalian Vps10p (vacuolar protein sorting 10 protein) domain family consisting of five transmembrane proteins with structural similarities: SorCS1, SorCS2, SorCS3, SorLA (sorting protein-related receptor with A-type repeats), and sortilin.[6] SorCS2 specifically has critical roles in neuronal viability and function. Single nucleotide polymorphisms (SNPs) in the protein has been associated with a range of diseases including attention-deficit hyperactivity disorder (ADHD),[7] bipolar disorders,[8] and schizophrenia,[9] and the receptor family has also been associated with Alzheimer's disease[10] and type 2 diabetes.[11]
Discovery
The Vps10p domain receptor family was based on the discovery of SorLA in 1996[12] and sortilin in 1997,[13] and has since been expanded with the SorCS subfamily with SorCS2 being described in 2001[14][15]
SorCS2 was first found from isolated cDNA in murine floor plate samples of the central nervous system (CNS) as well as in regions of the brain. The cDNA contained the characteristic Vps10p domain enabling its classification as a SorCS protein.[14] Not long after, a corresponding partial cDNA was found in human samples, and it was possible to determine the missing N-terminal by homology to murine SorCS2.[15]
Structure
SorCS2 is composed of a small intracellular region making a single pass into the extracellular environment where the large Vps10p domain make up a beta-propeller structure consisting of 10 propeller blade-like beta sheet regions. The Vps10p domain contains at least 2 unspecific ligand binding sites.[6] The domain also contains a furin cleavage site.[16] The extracellular region of SorCS proteins also include a LR (leucine rich domain) containing imperfect LR repeats (LRRs) which are known to serve as interaction and adhesion domains[15]
Modifications in Vps10p-type receptors include glycosylations.[17] and they also contain a propeptide which is proteolytically cleaved off to make them active[6]
In the non-neuronal glia cells, SorCS2 is cleaved and a linkage forms a two-chained product distinct from that in neurons which is a single chained. The processing in glia cells have been linked to proapoptotic properties not found in neuronal SorCS2.[18] This differential processing is thought to be common in Vps10p domain proteins where it regulates receptor functionality[6]
Dimerization
Efforts have been made to elucidate the structure of SorCS2, and this has allowed determination of dimerization of SorCS2 and the other two SorCS proteins with only few monomeric structures found. This dimerization is promoted by deglycosylation at least in SorCS1.[6] Structurally, the Vps10p domains in SorCS proteins can be found next to each other, but uniquely for SorCS2 it is prevalently found in a dimer where the domains are located away from each other and connected at a two-fold rotation axis for the dimer.[6] The different types of dimers could explain correspondingly different functions of SorCS2 found in different tissues. In addition to the homodimers described, the SorCS proteins also forms heterodimers within this subfamily.[6] Crystal structures of the full extracellular portion of SorCS2 have uncovered that SorCS2 consists of six domains.[19] Five domains contribute to the dimerization of SorCS2. Despite the extensive dimerization interface, SorCS2 has substantial conformational plasticity.[19]
Localization
SorCS2 and related proteins in the Vps10p domain family are predominantly found in neurons in the brain, but are also present in other tissues.[20] In terms of brain localization SorCS2 has been found predominantly in thalamus, floor plate of the midbrain and spinal cord, ventricular zones of hippocampal and accumbens areas, meninges, and Schwann cells. The localization is distinct from the other Vps10p receptor sortilin[21]
SorCS2 has further been found in tissues that are not brain related in smaller amounts e.g. in structures of mesodermal origin such as adipose tissue, striated muscle tissues, and developing bone as well as connective tissue such as the dermis, submucosal, and submesothelial tissues in the gut, and the bronchial system. Although the presence in these tissues are largely uninvestigated, they still form the basis for further specific functions in non-brain tissue.[20]
Function
All members of the Vps10p protein family are multiligand receptors.[22][23] They can take part in cellular trafficking and signaling through ligand binding in response to cellular conditions.[24][25][26] Examples of ligands are neurotrophic factors, amyloid precursor protein (APP), lipoproteins, and cytokines.[27] In addition to depending on the cellular context, the affinity for specific ligands can also be modulated by the monomer/dimer ratio.[6]
BDNF-dependent plasticity
Hippocampal N-methyl-D-aspartate (NMDA) receptor-dependent synaptic plasticity is found to be deficient at least in SorCS2 mutant mice, strongly suggesting a link between the two. SorCS2 deficient mice also show decreased long-term memory, higher tendency to take risks, and to have a more stimuli seeking behaviour than corresponding SorCS2 normal mice.[26]
The decrease in plasticity is attributed to the fact that SorCS2 forms a complex with p75NTR, a neurotrophin receptor which interacts with proBDNF (brain-derived neurotrophic factor) and TrkB (BDNF receptor tyrosine kinase) inside neurons in the hippocampal region of the brain to modulate synapse depression and potentiation respectively. Thus, SorCS2 could be the link between BDNF/proBDNF signaling and mental disorders. Deficiency in this signaling can affect the strengthening and weakening of synapses, that is, neuronal plasticity.[26]
Clinical significance
Alcohol withdrawal
When trying to stop excessive alcohol consumption alcohol withdrawal (AW) is physiological responses that in some cases can cause life-threatening seizures. SorCS2 has been associated with the severity of AW in genome analysis of European American test subjects, although no such connection could be made in African American samples[28]
A specific SorCS2 risk haplotype disrupts a transcription factor (TF) binding site in a stress hormone-modulated regulatory enhancer element with activity in human hippocampus. This region of the brain is already known for its association with AW. This increases the severity of AW in patients with alcoholism. Exposure to ethanol and glucocorticoids have been found to act as up-regulators of SorCS2, causing worsening of the problems if the risk variant of SorCS2 is present.[28]
See also
- SorCS1
- SorCS3
- Sortilin
- SorLA
- Vps10p domain
References
- GRCh38: Ensembl release 89: ENSG00000184985 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000029093 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "SORCS2 sortilin related VPS10 domain containing receptor 2 [Homo sapiens (human)] - Gene - NCBI". www.ncbi.nlm.nih.gov. Retrieved 2018-11-07.
- Januliene, Dovile; Manavalan, Arulmani; Ovesen, Peter Lund; Pedersen, Karen-Marie; Thirup, Søren; Nykjær, Anders; Moeller, Arne (September 2017). "Hidden Twins: SorCS Neuroreceptors Form Stable Dimers". Journal of Molecular Biology. 429 (19): 2907–2917. doi:10.1016/j.jmb.2017.08.006. ISSN 0022-2836. PMID 28827148.
- Alemany, Silvia; Ribasés, Marta; Vilor-Tejedor, Natàlia; Bustamante, Mariona; Sánchez-Mora, Cristina; Bosch, Rosa; Richarte, Vanesa; Cormand, Bru; Casas, Miguel (2015-07-14). "New suggestive genetic loci and biological pathways for attention function in adult attention-deficit/hyperactivity disorder". American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 168 (6): 459–470. doi:10.1002/ajmg.b.32341. ISSN 1552-4841. PMID 26174813. S2CID 38488226.
- Baum, A E; Akula, N; Cabanero, M; Cardona, I; Corona, W; Klemens, B; Schulze, T G; Cichon, S; Rietschel, M (2007-05-08). "A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder". Molecular Psychiatry. 13 (2): 197–207. doi:10.1038/sj.mp.4002012. ISSN 1359-4184. PMC 2527618. PMID 17486107.
- Christoforou, A; McGhee, K A; Morris, S W; Thomson, P A; Anderson, S; McLean, A; Torrance, H S; Le Hellard, S; Pickard, B S (2010-03-30). "Convergence of linkage, association and GWAS findings for a candidate region for bipolar disorder and schizophrenia on chromosome 4p". Molecular Psychiatry. 16 (3): 240–242. doi:10.1038/mp.2010.25. ISSN 1359-4184. PMID 20351716.
- Reitz, Christiane; Tokuhiro, Shinya; Clark, Lorraine N.; Conrad, Christopher; Vonsattel, Jean-Paul; Hazrati, Lili-Naz; Palotás, András; Lantigua, Raphael; Medrano, Martin (January 2011). "SORCS1 alters amyloid precursor protein processing and variants may increase Alzheimer's disease risk". Annals of Neurology. 69 (1): 47–64. doi:10.1002/ana.22308. ISSN 0364-5134. PMC 3086759. PMID 21280075.
- Goodarzi, Mark O.; Lehman, Donna M.; Taylor, Kent D.; Guo, Xiuqing; Cui, Jinrui; Quiñones, Manuel J.; Clee, Susanne M.; Yandell, Brian S.; Blangero, John (2007-07-01). "SORCS1: A Novel Human Type 2 Diabetes Susceptibility Gene Suggested by the Mouse". Diabetes. 56 (7): 1922–1929. doi:10.2337/db06-1677. ISSN 0012-1797. PMID 17426289.
- Jacobsen, L.; Madsen, P.; Moestrup, S. K.; Lund, A. H.; Tommerup, N.; Nykjaer, A.; Sottrup-Jensen, L.; Gliemann, J.; Petersen, C. M. (1996-12-06). "Molecular characterization of a novel human hybrid-type receptor that binds the alpha2-macroglobulin receptor-associated protein". The Journal of Biological Chemistry. 271 (49): 31379–31383. doi:10.1074/jbc.271.49.31379. ISSN 0021-9258. PMID 8940146.
- Petersen, C. M.; Nielsen, M. S.; Nykjaer, A.; Jacobsen, L.; Tommerup, N.; Rasmussen, H. H.; Roigaard, H.; Gliemann, J.; Madsen, P. (1997-02-07). "Molecular identification of a novel candidate sorting receptor purified from human brain by receptor-associated protein affinity chromatography". The Journal of Biological Chemistry. 272 (6): 3599–3605. doi:10.1074/jbc.272.6.3599. ISSN 0021-9258. PMID 9013611.
- Rezgaoui, M.; Hermey, G.; Riedel, I. B.; Hampe, W.; Schaller, H. C.; Hermans-Borgmeyer, I. (February 2001). "Identification of SorCS2, a novel member of the VPS10 domain containing receptor family, prominently expressed in the developing mouse brain". Mechanisms of Development. 100 (2): 335–338. doi:10.1016/S0925-4773(00)00523-2. ISSN 0925-4773. PMID 11165493. S2CID 18829884.
- Hampe, W.; Rezgaoui, M.; Hermans-Borgmeyer, I.; Schaller, H. C. (June 2001). "The genes for the human VPS10 domain-containing receptors are large and contain many small exons". Human Genetics. 108 (6): 529–536. doi:10.1007/s004390100504. ISSN 0340-6717. PMID 11499680. S2CID 23375354.
- Hosaka, M.; Nagahama, M.; Kim, W. S.; Watanabe, T.; Hatsuzawa, K.; Ikemizu, J.; Murakami, K.; Nakayama, K. (1991-07-05). "Arg-X-Lys/Arg-Arg motif as a signal for precursor cleavage catalyzed by furin within the constitutive secretory pathway". The Journal of Biological Chemistry. 266 (19): 12127–12130. doi:10.1016/S0021-9258(18)98867-8. ISSN 0021-9258. PMID 1905715.
- Hermey, Guido; Riedel, I.Björn; Hampe, Wolfgang; Schaller, H.Chica; Hermans-Borgmeyer, Irm (1999-12-20). "Identification and Characterization of SorCS, a Third Member of a Novel Receptor Family". Biochemical and Biophysical Research Communications. 266 (2): 347–351. doi:10.1006/bbrc.1999.1822. ISSN 0006-291X. PMID 10600506.
- Glerup, Simon; Olsen, Ditte; Vaegter, Christian B.; Gustafsen, Camilla; Sjoegaard, Susanne S.; Hermey, Guido; Kjolby, Mads; Molgaard, Simon; Ulrichsen, Maj (June 2014). "SorCS2 Regulates Dopaminergic Wiring and Is Processed into an Apoptotic Two-Chain Receptor in Peripheral Glia". Neuron. 82 (5): 1074–1087. doi:10.1016/j.neuron.2014.04.022. ISSN 0896-6273. PMID 24908487.
- Leloup, Nadia; Chataigner, Lucas M. P.; Janssen, Bert J. C. (2018-07-30). "Structural insights into SorCS2–Nerve Growth Factor complex formation". Nature Communications. 9 (1): 2979. Bibcode:2018NatCo...9.2979L. doi:10.1038/s41467-018-05405-z. ISSN 2041-1723. PMC 6065357. PMID 30061605.
- Boggild, Simon; Molgaard, Simon; Glerup, Simon; Nyengaard, Jens Randel (2016-03-10). "Spatiotemporal patterns of sortilin and SorCS2 localization during organ development". BMC Cell Biology. 17 (1): 8. doi:10.1186/s12860-016-0085-9. ISSN 1471-2121. PMC 4785631. PMID 26964886.
- Boggild, Simon; Molgaard, Simon; Glerup, Simon; Nyengaard, Jens Randel (2018-02-20). "Highly segregated localization of the functionally related vps10p receptors sortilin and SorCS2 during neurodevelopment". Journal of Comparative Neurology. 526 (8): 1267–1286. doi:10.1002/cne.24403. ISSN 0021-9967. PMID 29405286. S2CID 46869016.
- Willnow, Thomas E.; Petersen, Claus M.; Nykjaer, Anders (2008-11-12). "VPS10P-domain receptors — regulators of neuronal viability and function". Nature Reviews Neuroscience. 9 (12): 899–909. doi:10.1038/nrn2516. ISSN 1471-003X. PMID 19002190. S2CID 25776764.
- Willnow, Thomas E; Kjølby, Mads; Nykjaer, Anders (April 2011). "Sortilins: new players in lipoprotein metabolism". Current Opinion in Lipidology. 22 (2): 79–85. doi:10.1097/mol.0b013e3283416f2b. ISSN 0957-9672. PMID 21124217. S2CID 205829395.
- Nykjaer, Anders; Lee, Ramee; Teng, Kenneth K.; Jansen, Pernille; Madsen, Peder; Nielsen, Morten S.; Jacobsen, Christian; Kliemannel, Marco; Schwarz, Elisabeth (2004-02-26). "Sortilin is essential for proNGF-induced neuronal cell death". Nature. 427 (6977): 843–848. Bibcode:2004Natur.427..843N. doi:10.1038/nature02319. ISSN 0028-0836. PMID 14985763. S2CID 4343450.
- Larsen, Jakob Vejby; Hansen, Maria; Møller, Bjarne; Madsen, Peder; Scheller, Jürgen; Nielsen, Morten; Petersen, Claus Munck (2010-09-01). "Sortilin Facilitates Signaling of Ciliary Neurotrophic Factor and Related Helical Type 1 Cytokines Targeting the gp130/Leukemia Inhibitory Factor Receptor β Heterodimer". Molecular and Cellular Biology. 30 (17): 4175–4187. doi:10.1128/MCB.00274-10. ISSN 0270-7306. PMC 2937557. PMID 20584990.
- Glerup, S; Bolcho, U; Mølgaard, S; Bøggild, S; Vaegter, C B; Smith, A H; Nieto-Gonzalez, J L; Ovesen, P L; Pedersen, L F (2016-07-26). "SorCS2 is required for BDNF-dependent plasticity in the hippocampus". Molecular Psychiatry. 21 (12): 1740–1751. doi:10.1038/mp.2016.108. ISSN 1359-4184. PMID 27457814. S2CID 21763820.
- Glerup, S.; Nykjaer, A.; Vaegter, C. B. (2014), "Sortilins in Neurotrophic Factor Signaling", Neurotrophic Factors, Springer Berlin Heidelberg, vol. 220, pp. 165–189, doi:10.1007/978-3-642-45106-5_7, ISBN 9783642451058, PMID 24668473
- Smith, Andrew H.; Ovesen, Peter L.; Skeldal, Sune; Yeo, Seungeun; Jensen, Kevin P.; Olsen, Ditte; Diazgranados, Nancy; Zhao, Hongyu; Farrer, Lindsay A. (2018-10-25). "Risk Locus Identification Ties Alcohol Withdrawal Symptoms to SORCS2". Alcoholism: Clinical and Experimental Research. 42 (12): 2337–2348. doi:10.1111/acer.13890. ISSN 0145-6008. PMC 6317871. PMID 30252935.