Sodium dodecyl sulfate
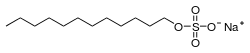
Sodium dodecyl sulfate (SDS) or sodium lauryl sulfate (SLS), sometimes written sodium laurilsulfate, is an organic compound with the formula CH3(CH2)11OSO3Na and structure H3C−(CH2)11−O−S(=O)2−O−Na+. It is an anionic surfactant used in many cleaning and hygiene products. This compound is the sodium salt of the 12-carbon organosulfate. Its hydrocarbon tail combined with a polar "headgroup" give the compound amphiphilic properties that make it useful as a detergent. SDS is also component of mixtures produced from inexpensive coconut and palm oils. SDS is a common component of many domestic cleaning, personal hygiene and cosmetic, pharmaceutical, and food products, as well as of industrial and commercial cleaning and product formulations.[2]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Sodium dodecyl sulfate | |
| Other names
Sodium monododecyl sulfate; Sodium lauryl sulfate; Sodium monolauryl sulfate; Sodium dodecanesulfate; dodecyl alcohol, hydrogen sulfate, sodium salt; n-dodecyl sulfate sodium; Sulfuric acid monododecyl ester sodium salt | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.005.263 |
| E number | E487 (thickeners, ...) |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H25NaSO4 | |
| Molar mass | 288.372 g/mol |
| Appearance | white or cream-colored solid |
| Odor | odorless |
| Density | 1.01 g/cm3 |
| Melting point | 206 °C (403 °F; 479 K) |
| Surface tension: | |
| 8.2 mM at 25 °C[1] | |
Refractive index (nD) |
1.461 |
| Pharmacology | |
| A06AG11 (WHO) | |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
1288 mg/kg (rat, oral) |
| Related compounds | |
Other anions |
Sodium laureth sulfate Sodium myreth sulfate |
Other cations |
Ammonium lauryl sulfate Potassium lauryl sulfate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Physicochemical properties

The critical micelle concentration (CMC) in water at 25 °C is 8.2 mM,[1] and the aggregation number at this concentration is usually considered to be about 62.[3] The micelle ionization fraction (α) is around 0.3 (or 30%).[4]
Applications
Cleaning and hygiene
SDS is mainly used in detergents for laundry with many cleaning applications.[5] It is a highly effective surfactant and is used in any task requiring the removal of oily stains and residues. For example, it is found in higher concentrations with industrial products including engine degreasers, floor cleaners, and car exterior cleaners.
It is a component in hand soap, toothpastes, shampoos, shaving creams, and bubble bath formulations, for its ability to create a foam (lather), for its surfactant properties, and in part for its thickening effect.[6]
Food additive
Sodium dodecyl sulfate, appearing as its synonym sodium lauryl sulfate (SLS), is considered a generally recognized as safe (GRAS) ingredient for food use according to the USFDA (21 CFR 172.822).[7] It is used as an emulsifying agent and whipping aid.[8] As an emulsifier in or with egg whites the United States Code of Federal Regulations require that it must not exceed 1,000 parts per million (0.1%) in egg white solids or 125 parts per million (0.0125%) in frozen or liquid egg whites and as a whipping agent for the preparation of marshmallows it must not exceed 0.5% of the weight of gelatine.[9] SLS is reported to temporarily diminish perception of sweetness.[10]
Laboratory applications
SDS is used in cleaning procedures,[11] and is commonly used as a component for lysing cells during RNA extraction and/or DNA extraction, and for denaturing proteins in preparation for electrophoresis in the SDS-PAGE technique.[12]

In the case of SDS-PAGE, the compound works by disrupting non-covalent bonds in the proteins, and so denaturing them, i.e. causing the protein molecules to lose their native conformations and shapes. By binding to proteins at a ratio of one SDS molecule per 2 amino acid residues, the negatively charged detergent provides all proteins with a similar net negative charge and therefore a similar charge-to-mass ratio.[13] In this way, the difference in mobility of the polypeptide chains in the gel can be attributed solely to their length as opposed to both their native charge and shape.[13][14] This separation based on the size of the polypeptide chain simplifies the analysis of protein molecules.[15]
Pharmaceutical applications
Sodium lauryl sulfate is a widely used in the pharmaceutical field as an ionic solubilizer and emulsifier that is suitable for applications in liquid dispersions, solutions, emulsions and micro emulsions, tablets, foams and semi-solids such as creams, lotions and gels.[16] Additionally, SLS aids in tablet wettability, as well as lubrication during manufacturing. Brand names of pharma-grade SLS include Kolliphor SLS and Kolliphor SLS Fine.[17]
Miscellaneous applications
SLS is used in an improved technique for preparing brain tissues for study by optical microscopy. The technique, which has been branded as CLARITY, was the work of Karl Deisseroth and coworkers at Stanford University, and involves infusion of the organ with an acrylamide solution to bind the macromolecules of the organ (proteins, nucleic acids, etc.), followed by thermal polymerization to form a "brain–hydrogel" (a mesh interspersed throughout the tissue to fix the macromolecules and other structures in space), and then by lipid removal using SDS to eliminate light scattering with minimal protein loss, rendering the tissue quasi-transparent.[18][19]
Along with sodium dodecylbenzene sulfonate and Triton X-100, aqueous solutions of SDS are popular for dispersing or suspending nanotubes, such as carbon nanotubes.[20]
Other uses
SLS has been proposed as a potentially effective topical microbicide, for intravaginal use, to inhibit and possibly prevent infection by various enveloped and non-enveloped viruses such as the herpes simplex viruses, HIV, and the Semliki Forest virus.[21][22]
Liquid membranes formed from SDS in water have been demonstrated to work as unusual particle separators.[23] The device acts as a reverse filter, allowing large particles to pass while capturing smaller particles.
Production
SDS is synthesized by treating lauryl alcohol with sulfur trioxide, oleum, or chlorosulfuric acid to produce hydrogen lauryl sulfate.[24] Lauryl alcohol can be used in pure form or as a mixtures of fatty alcohols. When produced from these sources, "SDS" products are a mixture of various sodium alkyl sulfates with SDS being the main component.[25] For instance, SDS is a component, along with other chain-length amphiphiles, when produced from coconut oil, and is known as sodium coco sulfate (SCS).[26] SDS is available commercially in powder, pellet, and other forms (each differing in rates of dissolution), as well as in aqueous solutions of varying concentrations.
Safety
SDS is not carcinogenic in low concentrations according to some studies.[27][28] Like all detergents, sodium lauryl sulfate removes oils from the skin, and can cause skin and eye irritation. It has been shown to irritate the skin of the face, with prolonged and constant exposure (more than an hour) in young adults.[29] SDS may worsen skin problems in individuals with chronic skin hypersensitivity, with some people being affected more than others.[30][31][32]
Oral concerns
SDS is a common ingredient in toothpastes due to its low cost,[33] its lack of impact on taste,[33] and its desirable action as a foaming agent.[33]
VSCs
SDS may reduce the amount of bad breath-causing volatile sulfur compounds (VSCs) in the mouth.[34] A series of small crossover studies (25–34 patients) have supported the efficacy of SLS in the reduction of VSCs, and its related positive impact on breath malodor, although these studies have been generally noted to reflect technical challenges in the control of study design variables.[34]
Dry mouth
Primary sources from the group of Irma Rantanen at University of Turku, Finland claim that SLS-containing pastes cause more dry mouth (xerostomia) than their proposed alternative. However, a 2011 Cochrane review of these studies, and of the more general area, concludes that there "is no strong evidence... that any topical therapy is effective for relieving the symptom of dry mouth."[35]
Mouth ulceration
A safety concern has been raised on the basis of several studies regarding the effect of toothpaste SDS on aphthous ulcers (more specifically, mouth ulcers or "canker sores"), commonly referred to as canker or white sores.[33] According to the NHS, SLS is a cause for concern for mouth ulcers.[36][37] As Lippert notes, of 2013, "very few... marketed toothpastes contain a surfactant other than SLS [SDS]," and leading manufacturers continue to formulate their produce with SDS.[33]
See also
- Sodium tetradecyl sulfate, another anionic surfactant in common use
- Mouth ulcer
References
- P. Mukerjee, P. & Mysels, K. J. (1971), "Critical Micelle Concentration of Aqueous Surfactant Systems," NSRDS-NBS 36, Washington, DC: US. Government Printing Office.
- Holmberg, Krister (2019). "Surfactants". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. pp. 1–56. doi:10.1002/14356007.a25_747.pub2.
- Turro, N.J.; Yekta, A. (1978). "Luminescent probes for detergent solutions. A simple procedure for determination of the mean aggregation number of micelles". J. Am. Chem. Soc. 100 (18): 5951–52. doi:10.1021/ja00486a062.
- Bales, Barney L.; Messina, Luis; Vidal, Arwen; Peric, Miroslav & Nascimento, Otaciro Rangel (1998). "Precision Relative Aggregation Number Determinations of SDS Micelles Using a Spin Probe. A Model of Micelle Surface Hydration". J. Phys. Chem. B. 102 (50): 10347–58. doi:10.1021/jp983364a.
- Smulders, Eduard ; Rybinski, Wolfgang; Sung, Eric; Rähse, Wilfried; Steber, Josef; Wiebel, Frederike & Nordskog, Anette. (2002) "Laundry Detergents," in Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a08_315.pub2
- "Household Products Database – Health and Safety Information on Household Products". nih.gov. Archived from the original on 12 June 2018. Retrieved 13 March 2016.
- "21 CFR 172.822 – Sodium lauryl sulfate". gpo.gov. Retrieved 13 March 2016.
- Igoe, R. S. (1983). Dictionary of food ingredients. New York: Van Nostrand Reinhold Co.
- "21 CFR 172.822 – Sodium lauryl sulfate". Retrieved 19 August 2021.
- Adams, Michael J. (1985). "Substances That Modify the Perception of Sweetness (Ch. 2)". In Bills, Donald D.; Mussinan, Cynthia J. (eds.). Characterization and Measurement of Flavor Compounds. ACS Symposium Series. Vol. 289. pp. 11–25. doi:10.1021/bk-1985-0289.ch002. ISBN 9780841209442.
- "Sodium Lauryl Sulfate – National Library of Medicine HSDB Database". toxnet.nlm.nih.gov. Retrieved 2017-02-16.
- The acronym expands to "sodium dodecyl sulfate-polyacrylamide gel electrophoresis."
- Janson, Lee W., 1964- (2012). The big picture : medical biochemistry. Tischler, Marc E. New York: McGraw-Hill. ISBN 978-0-07-163792-3. OCLC 794620168.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Ninfa, Alexander; Ballou, David; Benore, Marilee (2009). Fundamental Laboratory Approaches for Biochemistry and Biotechnology. United States: Wiley, John and Sons, Incorporated. p. 165. ISBN 978-0470087664.
- Ninfa, Alexander; Ballou, David (1998). Fundamental Laboratory Approaches for Biochemistry and Biotechnology. Hoboken, New Jersey: John Wiley & Sons. p. 129. ISBN 978-1-891-78600-6.
- "Pharmaceuticals". pharmaceutical.basf.com. Retrieved 2021-04-27.
- "Kolliphor® SLS". pharmaceutical.basf.com. Retrieved 2021-04-27.
- Shen, Helen (2013). "See-through brains clarify connections". Nature. 496 (7444, April 10): 151. Bibcode:2013Natur.496..151S. doi:10.1038/496151a. PMID 23579658.
- Chung, K.; Wallace, J.; Kim, S.-Y.; et al. (2013). "Structural and molecular interrogation of intact biological systems". Nature. 497 (7449, May 16): 332–37. Bibcode:2013Natur.497..332C. doi:10.1038/nature12107. PMC 4092167. PMID 23575631.
Obtaining high-resolution information from a complex system, while maintaining the global perspective needed to understand system function, represents a key challenge in biology. Here we address this challenge with a method (termed CLARITY) for the transformation of intact tissue into a nanoporous hydrogel-hybridized form (crosslinked to a three-dimensional network of hydrophilic polymers) that is fully assembled but optically transparent and macromolecule-permeable.
- Islam, M. F. (2003). "High Weight Fraction Surfactant Solubilization of Single-Wall Carbon Nanotubes in Water". Nano Letters. 3 (2): 269–73. Bibcode:2003NanoL...3..269I. doi:10.1021/nl025924u.
- Piret J.; Désormeaux, A. & Bergeron, M.G. (2002). "Sodium lauryl sulfate, a microbicide effective against enveloped and nonenveloped viruses". Curr. Drug Targets. 3 (1): 17–30. doi:10.2174/1389450023348037. PMID 11899262.
- Piret J.; Lamontagne, J.; Bestman-Smith, J.; Roy, S.; Gourde, P.; Désormeaux, A.; Omar, R.F.; Juhász, J. & Bergeron, M.G. (2000). "In vitro and in vivo evaluations of sodium lauryl sulfate and dextran sulfate as microbicides against herpes simplex and human immunodeficiency viruses". J. Clin. Microbiol. 38 (1): 110–19. doi:10.1128/JCM.38.1.110-119.2000. PMC 86033. PMID 10618073.
- Birgitt Boschitsch Stogin; et al. (August 24, 2018). "Free-standing liquid membranes as unusual particle separators". Science Advances. 4 (8): eaat3276. Bibcode:2018SciA....4.3276S. doi:10.1126/sciadv.aat3276. PMC 6108570. PMID 30151426.
- Takei, Kensuke; Tsuto, Keiichi; Miyamoto, Shigeyuki; Wakatsuki, Junya (February 1985). "Anionic surfactants: lauric products". Journal of the American Oil Chemists' Society. 62 (2): 341–347. doi:10.1007/BF02541402. S2CID 84286689.
- Gloxhuber, C., & Kunster, K. (1992). Anionic Surfactants: Biochemistry, toxicology, dermatology (2nd ed.). New York.
{{cite book}}: CS1 maint: location missing publisher (link) CS1 maint: multiple names: authors list (link) - US 3,491,033, "Process of making solid foams from polymer emulsions", published 1970
- Cosmetic Ingredient Review (CIR) program Expert Panel (1983). "Final Report on the Safety Assessment of Sodium Lauryl Sulfate and Ammonium Lauryl Sulfate" (PDF). Int. J. Toxicol. 2 (7): 127–81. doi:10.3109/10915818309142005. S2CID 34123578. Retrieved 13 March 2016.
[Quoting:] Carcinogenesis. A one-year chronic oral study using beagles showed that Sodium Lauryl Sulfate at concentrations up to 2% in the diet was not tumorigenic or carcinogenic. [p. 157] / Summary… In mutagenesis studies, rats fed 1.13% and 0.56% Sodium Lauryl Sulfate in the diet for 90 days produced no more chromosomal aberrations or clastogenic effects than did a control diet. [p. 175]. / Conclusion. Sodium Lauryl Sulfate and Ammonium Lauryl Sulfate appear to be safe in formulations designed for discontinuous, brief use followed by thorough rinsing from the surface of the skin. In products intended for prolonged contact with skin, concentrations should not exceed 1%. [p. 176.]
. - Wilma F. Bergfeld, Chair, and the Cosmetic Ingredient Review (CIR) program Expert Panel (2005). "Final report on the safety assessment of sodium lauryl sulfate and ammonium lauryl sulfate" (PDF). Int. J. Toxicol. 24 (1): 1–102, esp. 89–98. Retrieved 13 March 2016.
[Quoting:] Sodium Lauryl Sulfate and Ammonium Lauryl Sulfate appear to be safe in formulations designed for discontinuous, brief use followed by thorough rinsing from the surface of the skin. In products intended for prolonged contact with skin, concentrations should not exceed 1%… New studies confirmed the irritant properties of these ingredients and reinforced the concentration limit of 1% or leave-on uses established by the [earlier] Panel. [p. 89] / The available studies that looked for carcinogenesis failed to find evidence that Ammonium Lauryl Sulfate are [sic.] carcinogenic. None of the available data suggested that SLS or Ammonium Lauryl Sulfate could be carcinogenic. Despite assertions to the contrary on the Internet, the carcinogenicity of these ingredients is only a rumor. [pp. 89ff]
{{cite journal}}: CS1 maint: multiple names: authors list (link). - Marrakchi S, Maibach HI (2006). "Sodium lauryl sulfate-induced irritation in the human face: regional and age-related differences". Skin Pharmacol Physiol. 19 (3): 177–80. doi:10.1159/000093112. PMID 16679819. S2CID 35890797.
- Agner T (1991). "Susceptibility of atopic dermatitis patients to irritant dermatitis caused by sodium lauryl sulphate". Acta Derm. Venereol. 71 (4): 296–300. doi:10.2340/0001555571296300. PMID 1681644. S2CID 37806228.
- Nassif A, Chan SC, Storrs FJ, Hanifin JM (November 1994). "Abnormal skin irritancy in atopic dermatitis and in atopy without dermatitis". Arch Dermatol. 130 (11): 1402–07. doi:10.1001/archderm.130.11.1402. PMID 7979441.
- Löffler H, Effendy I (May 1999). "Skin susceptibility of atopic individuals". Contact Derm. 40 (5): 239–42. doi:10.1111/j.1600-0536.1999.tb06056.x. PMID 10344477. S2CID 10409476.
- Lippert, Frank (2013). "An Introduction to Toothpaste—Its Purpose, History and Ingredients". In van Loveren, Cor (ed.). Toothpastes. Monographs in Oral Science. Vol. 23. Series Eds.: Huysmans, M.C., Lussi, A. & Weber, H.-P. Basel, CHE: Karger. pp. 1–14, esp. 12. doi:10.1159/000350456. ISBN 978-3-318-02206-3. PMID 23817056.
- Dadamio, J.; Laleman, I. & Quirynen, M. (2013). "The Role of Toothpastes in Oral Malodor Management". In van Loveren, C. (ed.). Toothpastes. Monographs in Oral Science. Vol. 23. Series Eds.: Huysmans, M.C., Lussi, A. & Weber, H.-P. Basel, CHE: Karger. pp. 45–60, esp. 49–52. doi:10.1159/000350472. ISBN 978-3-318-02206-3. PMID 23817059.
- See Furness S.; Worthington, H.V.; Bryan, G.; Birchenough, S. & McMillan R. (2011). "Interventions for the management of dry mouth: topical therapies". Cochrane Database Syst Rev. 7 (12, December): CD008934. doi:10.1002/14651858.CD008934.pub2. PMID 22161442.
[Quoting abstract:] There is no strong evidence from this review that any topical therapy is effective for relieving the symptom of dry mouth.
See Rantanen, et al. (2003) J. Contemp. Dent. Pract. 4(2):11–23, , and Rantanen, et al. (2003) Swed. Dent. J. 27(1):31–34, , referenced therein. - "Mouth ulcers". NHS. 18 October 2017.
do not use toothpaste containing sodium lauryl sulphate
- Some of the published studies, from latest to earliest, are as follows. (i) A 2012 double-blind crossover study of 90-patients failed to find a significant difference in number of ulcers between groups using SLS-containing toothpaste, versus a group using an SLS-free toothpaste, but did suggest significant reduction in ulcer duration and improvement in patient pain scores, see Shim, Y. J.; Choi, J. -H.; Ahn, H. -J.; Kwon, J. -S. (2012). "Effect of sodium lauryl sulfate on recurrent aphthous stomatitis: A randomized controlled clinical trial". Oral Diseases. 18 (7): 655–60. doi:10.1111/j.1601-0825.2012.01920.x. PMID 22435470., a study also cited in the Lippert (2013) book chapter. (ii) A 1999 double-blind crossover study of 47 patients failed to find any statistically significant difference in the number, episodes, and duration of such ulcers between these two groups, and of pain scores between them, see Healy CM, Paterson M, Joyston-Bechal S, Williams DM, Thornhill MH (January 1999). "The effect of a sodium lauryl sulfate-free dentifrice on patients with recurrent oral ulceration". Oral Dis. 5 (1): 39–43. doi:10.1111/j.1601-0825.1999.tb00062.x. PMID 10218040. (iii) A 1997 study suggested a significantly higher number of ulcers after SLS toothpaste use, versus its control group, see Chahine L, Sempson N, Wagoner C (December 1997). "The effect of sodium lauryl sulfate on recurrent aphthous ulcers: a clinical study". Compend. Contin. Educ. Dent. 18 (12): 1238–40. PMID 9656847., a study also cited in the Lippert (2013) book chapter. (iv) A 1996 follow-up 30-patient double-blind crossover study and a 1994 preliminary 10-patient crossover study by the same authors suggested significantly higher numbers of aphthous ulcers after using SLS-containing toothpaste, compared with an SLS-free toothpaste, see Herlofson BB, Barkvoll P (June 1996). "The effect of two toothpaste detergents on the frequency of recurrent aphthous ulcers". Acta Odontol. Scand. 54 (3): 150–53. doi:10.3109/00016359609003515. PMID 8811135. and Herlofson BB, Barkvoll P (October 1994). "Sodium lauryl sulfate and recurrent aphthous ulcers. A preliminary study". Acta Odontol. Scand. 52 (5): 257–59. doi:10.3109/00016359409029036. PMID 7825393.