Fast atom bombardment
Fast atom bombardment (FAB) is an ionization technique used in mass spectrometry in which a beam of high energy atoms strikes a surface to create ions.[1][2][3] It was developed by Michael Barber at the University of Manchester in 1980.[4] When a beam of high energy ions is used instead of atoms (as in secondary ion mass spectrometry), the method is known as liquid secondary ion mass spectrometry (LSIMS).[5][6][7] In FAB and LSIMS, the material to be analyzed is mixed with a non-volatile chemical protection environment, called a matrix, and is bombarded under vacuum with a high energy (4000 to 10,000 electron volts) beam of atoms. The atoms are typically from an inert gas such as argon or xenon. Common matrices include glycerol, thioglycerol, 3-nitrobenzyl alcohol (3-NBA), 18-crown-6 ether, 2-nitrophenyloctyl ether, sulfolane, diethanolamine, and triethanolamine. This technique is similar to secondary ion mass spectrometry and plasma desorption mass spectrometry.
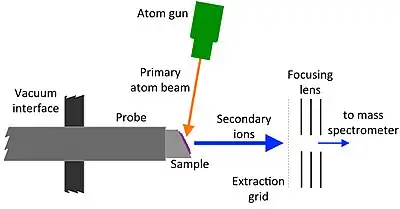
Ionization mechanism
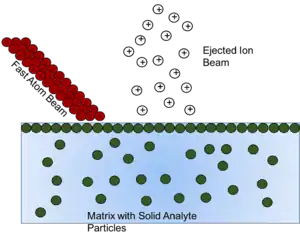
FAB is a relatively low fragmentation (soft) ionization technique and produces primarily intact protonated molecules denoted as [M + H]+ and deprotonated molecules such as [M - H]−. Radical cations can also be observed in a FAB spectrum in rare cases. FAB was designed as an improved version of SIMS that allowed for the primary beam to no longer cause damaging effects to the sample. The major difference between the two techniques is the difference in the nature of the primary beam used; ions vs atoms.[8] For LSIMS, Cesium, Cs+ ions make up the primary beam and for FAB the primary beam is made up of Xe or Ar atoms.[8] Xe atoms are used because they tend to be more sensitive than Argon atoms due to their larger masses and more momentum. For the molecules to be ionized by FAB, first the slow moving atoms (Xe or Ar) are ionized by colliding electrons. Those slow moving atoms are then ionized and accelerated to a certain potential where they develop into fast moving ions that become neutral in a dense cloud of excess natural gas atoms that make a flowing stream of high translational energy atoms.[8] Although the exact mechanism of how the samples are ionized have not been fully discovered, the nature of its ionization mechanism is similar to matrix-assisted laser desorption/ionization (MALDI)[9][10] and chemical ionization.[11]
Matrices and sample introduction
As previously stated, in FAB the samples are mixed with a non-volatile environment (matrix) in order to be analyzed. FAB uses a liquid matrix that is mixed with the sample in order to provide a sample ion current that is sustained, reduces damages made to the sample by absorbing the impact of the primary beam, and keeps the sample molecules form aggregating.[8] The liquid matrix, like any other matrix, most importantly provides a medium that promotes sample ionization. The most widely accepted matrix for this type of ionization is glycerol. Choosing the appropriate matrix for the sample is crucial because the matrix can also influence the degree of fragmentation of the sample (analyte) ions. The sample can then be introduced to FAB analysis. The normal method of introducing the sample-matrix mixture is through an insertion probe. The sample-matrix mixture is loaded on a stainless steel sample target on the probe, which is then placed in the ion source via a vacuum lock. The alternative method of introducing the sample is by using a device called continuous flow fast atom bombardment (CF)-FAB.
Continuous flow fast atom bombardment
In continuous flow fast atom bombardment (CF-FAB), the sample is introduced into the mass spectrometer insertion probe through a small diameter capillary.[12] (CF)-FAB was developed to minimize the problem of poor detection sensitivity that is caused by an excess of the matrix background that results in a high matrix-to-sample ratio.[8] When a metal frit is used to disperse the liquid on the probe, the technique is known as frit FAB.[13][14] Samples can be introduced by flow injection, microdialysis, or by coupling with liquid chromatography.[15] Flow rates are typically between 1 and 20 μL/min.[13] CF-FAB has a higher sensitivity compared to static FAB[16]
Applications

The first example of the practical application of this FAB was the elucidation of the amino acid sequence of the oligopeptide efrapeptin D. This contained a variety of very unusual amino acid residues.[17] The sequence was shown to be: N-acetyl-L-pip-AIB-L-pip-AIB-AIB-L-leu-beta-ala-gly-AIB-AIB-L-pip-AIB-gly-L-leu-L-iva-AIB-X. PIP = pipecolic acid, AIB = alpha-amino-isobutyric acid, leu = leucine, iva = isovaline, gly = glycine. This is a potent inhibitor of mitochondrial ATPase activity. Another application of FAB includes its original use for the analysis of condensed-phase samples. FAB can be use for measurements of the molecular weight of samples below 5000 Da, as well as their structural characteristics. FAB can be paired with various mass spectrometers for data analysis, such as with a quadrupole mass analyzer, liquid chromatography–mass spectrometry, and more.
Inorganic analysis
In 1983 a paper was published describing the use of fast atom bombardment mass spectrometry (FAB-MS) to analyze isotopes of calcium.[18] Glycerol was not used; samples in aqueous solution were deposited on the sample target and dried prior to analysis. The technique was effectively secondary ion mass spectrometry using a neutral primary beam. This was a welcomed development for biomedical researchers studying the nutrition and metabolism of essential minerals but lacking access to inorganic mass spectrometry instrumentation such as thermal ionization mass spectrometry or inductively-coupled plasma mass spectrometry (ICP-MS). In contrast, FAB mass spectrometers were widely found in biomedical research institutions. Multiple laboratories adopted this technique, using FAB-MS to measure isotope ratios in isotope tracer studies of calcium, iron, magnesium and zinc.[19] The analysis of metals required minimal modification of the mass spectrometers, e.g.replacing the stainless steel sample targets with pure silver ones to eliminate background from ionization of stainless steel components.[20] Signal acquisition systems were sometimes modified to perform peak jumping instead of scanning and to do ion counting detection.[21] While satisfactory precision and accuracy were attained with FAB-MS, the technique was labor-intensive with a very low sample through-put rate due in part to the absence of auto-sampling options.[19] By the early 2000s this severe sampling rate limitation had motivated users of FAB-MS for mineral isotope analysis to switch to conventional inorganic mass spectrometers, usually ICP-MS which also exhibited improved affordability and isotope ratio analysis performance by that time.
References
- Morris HR, Panico M, Barber M, Bordoli RS, Sedgwick RD, Tyler A (1981). "Fast atom bombardment: a new mass spectrometric method for peptide sequence analysis". Biochem. Biophys. Res. Commun. 101 (2): 623–31. doi:10.1016/0006-291X(81)91304-8. PMID 7306100.
- Barber, Michael; Bordoli, Robert S.; Elliott, Gerard J.; Sedgwick, R. Donald; Tyler, Andrew N. (1982). "Fast Atom Bombardment Mass Spectrometry". Analytical Chemistry. 54 (4): 645A–657A. doi:10.1021/ac00241a817. ISSN 0003-2700.
- Barber M, Bordoli RS, Sedgewick RD, Tyler AN (1981). "Fast atom bombardment of solids (F.A.B.): a new ion source for mass spectrometry". Journal of the Chemical Society, Chemical Communications (7): 325–7. doi:10.1039/C39810000325.
- Barber, M.; Bordoli, R. S.; Sedgwick, R. D.; Tyler, A. N. (September 1981). "Fast atom bombardment of solids as an ion source in mass spectrometry". Nature. 293 (5830): 270–275. doi:10.1038/293270a0. ISSN 0028-0836.
- Stoll, R.G.; Harvan, D.J.; Hass, J.R. (1984). "Liquid secondary ion mass spectrometry with a focussed primary ion source". International Journal of Mass Spectrometry and Ion Processes. 61 (1): 71–79. Bibcode:1984IJMSI..61...71S. doi:10.1016/0168-1176(84)85118-6. ISSN 0168-1176.
- Dominic M. Desiderio (14 November 1990). Mass Spectrometry of Peptides. CRC Press. pp. 174–. ISBN 978-0-8493-6293-4.
- De Pauw, E.; Agnello, A.; Derwa, F. (1991). "Liquid matrices for liquid secondary ion mass spectrometry-fast atom bombardment: An update". Mass Spectrometry Reviews. 10 (4): 283–301. Bibcode:1991MSRv...10..283D. doi:10.1002/mas.1280100402. ISSN 0277-7037.
- Chhabil., Dass (2007-01-01). Fundamentals of contemporary mass spectrometry. Wiley-Interscience. ISBN 9780471682295. OCLC 609942304.
- Pachuta, Steven J.; Cooks, R. G. (1987). "Mechanisms in molecular SIMS". Chemical Reviews. 87 (3): 647–669. doi:10.1021/cr00079a009. ISSN 0009-2665.
- Tomer KB (1989). "The development of fast atom bombardment combined with tandem mass spectrometry for the determination of biomolecules". Mass Spectrometry Reviews. 8 (6): 445–82. Bibcode:1989MSRv....8..445T. doi:10.1002/mas.1280080602.
- Székely, Gabriella; Allison, John (1997). "If the ionization mechanism in fast-atom bombardment involves ion/molecule reactions, what are the reagent ions? The time dependence of fast-atom bombardment mass spectra and parallels to chemical ionization". Journal of the American Society for Mass Spectrometry. 8 (4): 337–351. doi:10.1016/S1044-0305(97)00003-2. ISSN 1044-0305.
- Caprioli, Richard M. (1990). "Continuous-flow fast atom bombardment mass spectrometry". Analytical Chemistry. 62 (8): 477A–485A. doi:10.1021/ac00207a715. ISSN 0003-2700. PMID 2190496.
- Jürgen H Gross (14 February 2011). Mass Spectrometry: A Textbook. pp. 494–. Bibcode:2005PhT....58f..59G. doi:10.1063/1.1996478. ISBN 978-3-642-10709-2.
{{cite book}}:|journal=ignored (help) - Caprioli, R. M. (1990). Continuous-flow fast atom bombardment mass spectrometry. New York: Wiley. ISBN 978-0-471-92863-8.
- Abian, J. (1999). "The coupling of gas and liquid chromatography with mass spectrometry". Journal of Mass Spectrometry. 34 (3): 157–168. Bibcode:1999JMSp...34..157A. doi:10.1002/(SICI)1096-9888(199903)34:3<157::AID-JMS804>3.0.CO;2-4. ISSN 1076-5174.
- Tomer, K. B.; Perkins, J. R.; Parker, C. E.; Deterding, L. J. (1991-12-01). "Coaxial continuous flow fast atom bombardment for higher-molecular-weight peptides: comparison with static fast atom bombardment and electrospray ionization". Biological Mass Spectrometry. 20 (12): 783–788. doi:10.1002/bms.1200201207. ISSN 1052-9306. PMID 1812988.
- Bullough,D.A., Jackson C.G.,Henderson, P.J.F., Cottee, F.H.,Beechey,R.B. and Linnett, P.E. Biochemistry International (1981) 4, 543-549
- Smith, David (December 1983). "Determination of Stable Isotopes of Calcium in Biological Fluids by Fast Atom Bombardment Mass Spectrometry". Analytical Chemistry. 55: 2391–2393.
- Eagles, John; Mellon, Fred (1996). "Chapter 10: Fast Atom Bombardment Mass Spectrometry (FABMS)". In Mellon, Fred; Sandstrom, Britmarie (eds.). Stable Isotopes in Human Nutrition:Inorganic Nutrient Metabolism. London: Academic Press. pp. 73–80. ISBN 0-12-490540-4.
- Miller, Leland; Hambidge, Michael; Fennessey, Paul (1991). "Isotope Fractionation and Hydride Interference in Metal Isotope Analysis by Fast Atom Bombardment-Induced Secondary Ion Mass Spectrometry". Journal of Micronutrient Analysis. 8: 179–197.
- Krebs, Nancy; Miller, Leland; Naake, Vernon; Lei, Sian; Westcott, Jamie; Fennessey, Paul; Hambidge, Michael (June 1995). "The Use of Stable Isotope Techniques to Assess Zinc Metabolism". Nutritional Biochemistry. 6: 292–301.