Strontium lactate
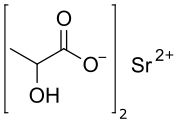
Strontium lactate is a chemical compound, a salt of strontium and lactic acid with the formula C
6H
10O
6Sr.[1][2] This salt is stable and non-radioactive.[3]
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.045.363 |
| EC Number |
|
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C 6H 10O 6Sr | |
| Molar mass | 265.76 |
| Appearance | white powder |
| Soluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Synthesis
Strontium lactate can be obtained by neutralizing moderately dilute lactic acid with strontium carbonate or hydroxide and evaporating the resulting solution to dryness with a moderate heat.[4]
Use
Strontium lactate is used as a dietary supplement for treating osteoporosis and supporting bones and teeth.[5][6]
References
- "Strontium lactate". National Institute of Standards and Technology. 17 October 2019. Retrieved 6 February 2023.
- "NCATS Inxight Drugs — STRONTIUM LACTATE". drugs.ncats.io. Retrieved 6 February 2023.
- MacDonald, Norman S.; Nusbaum, Ralph E.; Stearns, Richard.; Ezmirlian, Florita.; McArthur, Clare.; Spain, Patricia. (1951). "The Skeletal Deposition of Non-Radioactive Strontium". Journal of Biological Chemistry. 188 (1): 137–143. doi:10.1016/S0021-9258(18)56154-8. PMID 14814122.
- Caspari, Charles (1895). A Treatise on pharmacy for students and pharmacists. Lea. p. 477. Retrieved 6 February 2023.
- "Strontium Lactate - 29870-99-3". Discovery Fine Chemicals. Retrieved 6 February 2023.
- "Safety and Pharmacokinetics of Orally Administered Strontium L-Lactate in Healthy Adults". clinicaltrials.gov. 30 November 2018. Retrieved 6 February 2023.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.