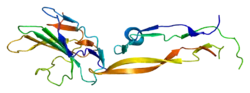TGF beta receptor 2
Transforming growth factor, beta receptor II (70/80kDa) is a TGF beta receptor. TGFBR2 is its human gene.
It is a tumor suppressor gene.[5]
Function
This gene encodes a member of the serine/threonine protein kinase family and the TGFB receptor subfamily. The encoded protein is a transmembrane protein that has a protein kinase domain, forms a heterodimeric complex with another receptor protein, and binds TGF-beta. This receptor/ligand complex phosphorylates proteins, which then enter the nucleus and regulate the transcription of a subset of genes related to cell proliferation. Mutations in this gene have been associated with Marfan syndrome, Loeys-Deitz aortic aneurysm syndrome, Osler–Weber–Rendu syndrome, and the development of various types of tumors. At least 73 disease-causing mutations in this gene have been discovered.[6] Alternatively spliced transcript variants encoding different isoforms have been characterized.[7]
Interactions
TGF beta receptor 2 has been shown to interact with:
Domain architecture
| Transforming growth factor beta receptor 2 ectodomain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
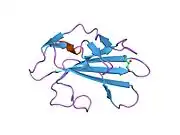 crystal structure of human tgf-beta type ii receptor ligand binding domain | |||||||||
| Identifiers | |||||||||
| Symbol | ecTbetaR2 | ||||||||
| Pfam | PF08917 | ||||||||
| InterPro | IPR015013 | ||||||||
| |||||||||
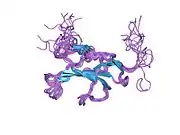
TGF beta receptor 2 consists of a C-terminal protein kinase domain and an N-terminal ectodomain. The ectodomain consists of a compact fold containing nine beta-strands and a single helix stabilised by a network of six intra strand disulphide bonds. The folding topology includes a central five-stranded antiparallel beta-sheet, eight-residues long at its centre, covered by a second layer consisting of two segments of two-stranded antiparallel beta-sheets (beta1-beta4, beta3-beta9).[18]
See also
References
- GRCh38: Ensembl release 89: ENSG00000163513 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000032440 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "TGFBR2 - transforming growth factor, beta receptor II (70/80kDa) - Genetics Home Reference". Archived from the original on 2011-08-10. Retrieved 2008-09-07.
- Šimčíková D, Heneberg P (December 2019). "Refinement of evolutionary medicine predictions based on clinical evidence for the manifestations of Mendelian diseases". Scientific Reports. 9 (1): 18577. Bibcode:2019NatSR...918577S. doi:10.1038/s41598-019-54976-4. PMC 6901466. PMID 31819097.
- "Entrez Gene: TGFBR2 transforming growth factor, beta receptor II (70/80kDa)".
- Yao D, Ehrlich M, Henis YI, Leof EB (Nov 2002). "Transforming growth factor-beta receptors interact with AP2 by direct binding to beta2 subunit". Molecular Biology of the Cell. 13 (11): 4001–12. doi:10.1091/mbc.02-07-0104. PMC 133610. PMID 12429842.
- Liu JH, Wei S, Burnette PK, Gamero AM, Hutton M, Djeu JY (Jan 1999). "Functional association of TGF-beta receptor II with cyclin B". Oncogene. 18 (1): 269–75. doi:10.1038/sj.onc.1202263. PMID 9926943.
- Barbara NP, Wrana JL, Letarte M (Jan 1999). "Endoglin is an accessory protein that interacts with the signaling receptor complex of multiple members of the transforming growth factor-beta superfamily". The Journal of Biological Chemistry. 274 (2): 584–94. doi:10.1074/jbc.274.2.584. PMID 9872992.
- Guerrero-Esteo M, Sanchez-Elsner T, Letamendia A, Bernabeu C (Aug 2002). "Extracellular and cytoplasmic domains of endoglin interact with the transforming growth factor-beta receptors I and II". The Journal of Biological Chemistry. 277 (32): 29197–209. doi:10.1074/jbc.M111991200. PMID 12015308.
- Wrighton KH, Lin X, Feng XH (Jul 2008). "Critical regulation of TGFbeta signaling by Hsp90". Proceedings of the National Academy of Sciences of the United States of America. 105 (27): 9244–9. Bibcode:2008PNAS..105.9244W. doi:10.1073/pnas.0800163105. PMC 2453700. PMID 18591668.
- Datta PK, Chytil A, Gorska AE, Moses HL (Dec 1998). "Identification of STRAP, a novel WD domain protein in transforming growth factor-beta signaling". The Journal of Biological Chemistry. 273 (52): 34671–4. doi:10.1074/jbc.273.52.34671. PMID 9856985.
- Datta PK, Moses HL (May 2000). "STRAP and Smad7 synergize in the inhibition of transforming growth factor beta signaling". Molecular and Cellular Biology. 20 (9): 3157–67. doi:10.1128/mcb.20.9.3157-3167.2000. PMC 85610. PMID 10757800.
- Kawabata M, Chytil A, Moses HL (Mar 1995). "Cloning of a novel type II serine/threonine kinase receptor through interaction with the type I transforming growth factor-beta receptor". The Journal of Biological Chemistry. 270 (10): 5625–30. doi:10.1074/jbc.270.10.5625. PMID 7890683.
- Razani B, Zhang XL, Bitzer M, von Gersdorff G, Böttinger EP, Lisanti MP (Mar 2001). "Caveolin-1 regulates transforming growth factor (TGF)-beta/SMAD signaling through an interaction with the TGF-beta type I receptor". The Journal of Biological Chemistry. 276 (9): 6727–38. doi:10.1074/jbc.M008340200. PMID 11102446.
- De Crescenzo G, Pham PL, Durocher Y, O'Connor-McCourt MD (May 2003). "Transforming growth factor-beta (TGF-beta) binding to the extracellular domain of the type II TGF-beta receptor: receptor capture on a biosensor surface using a new coiled-coil capture system demonstrates that avidity contributes significantly to high affinity binding". Journal of Molecular Biology. 328 (5): 1173–83. doi:10.1016/s0022-2836(03)00360-7. PMID 12729750.
- Hart PJ, Deep S, Taylor AB, Shu Z, Hinck CS, Hinck AP (Mar 2002). "Crystal structure of the human TbetaR2 ectodomain--TGF-beta3 complex". Nature Structural Biology. 9 (3): 203–8. doi:10.1038/nsb766. PMID 11850637. S2CID 13322593.
- Rotzer D, Roth M, Lutz M, Lindemann D, Sebald W, Knaus P (Feb 2001). "Type III TGF-beta receptor-independent signalling of TGF-beta2 via TbetaRII-B, an alternatively spliced TGF-beta type II receptor". The EMBO Journal. 20 (3): 480–90. doi:10.1093/emboj/20.3.480. PMC 133482. PMID 11157754.