Tail flick test
The tail flick test is a test of the pain response in animals, similar to the hot plate test. It is used in basic pain research and to measure the effectiveness of analgesics, by observing the reaction to heat. It was first described by D'Amour and Smith in 1941.[1]
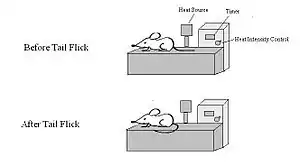
Procedure
Most commonly, an intense light beam is focused on the animal's tail and a timer starts. When the animal flicks its tail, the timer stops and the recorded time (latency) is a measure of the pain threshold.[2] Alternate methods can be used to apply heat, such as immersion in hot water.[3]
Alternately, a dolorimeter with a resistance wire with a constant heat flow may be used. For the tail flick test, the wire is attached to the tail of the organism, and the wire applies heat to the tail. The researcher then records the latency to tail flick.[4]
Applications
Researchers testing the effectiveness of drugs on the pain threshold often use the tail flick test to measure the extent to which the drug being tested has reduced the amount of pain felt by the model organism.[5]
Both laboratory mice and rats are a common model organism for these tests. These rodents are usually given analgesics, which are responsible for weakening the response to pain. Under these weakened responses to pain, with effectiveness often peaking about 30 minutes after ingestion, researchers test the effectiveness of the drugs by exposing the tail to constant heat and measuring how long it takes to flick, signaling its response to the pain.[6][7] Naloxone and naltrexone, two opioid antagonists, have been used to study pain sensitivity in relation to exercise in mice.[8]
Experimental tests of the tail flick testing method showed that the temperature of the skin of the tail plays a major role in the critical temperature, i.e., the temperature at which the tail flicks in response to pain. Researchers found that if the tail has been exposed to a cooler temperatures before the test, then the critical temperature decreases.[9]
Through use of the tail flick test, researchers have found that genetics play a role in pain sensation and the effectiveness of analgesics. A mouse of one genetic line may be more or less tolerant of pain than a mouse of another genetic line. Also, a mouse of one genetic line may experience a higher or lower effectiveness of an analgesic than a mouse of another genetic line. Using this test, researchers can also begin to identify genes that play a role in pain sensation. For example, the Calca gene (see WikiGenes CALCA) is primarily responsible for the variability in thermal (heat) nociception.[10] The Sprawling mutation (see WikiGenes Swl) resulted in a moderate sensory neuropathy but the mutation did not affect nociceptive modality or motor function in the mice. The mice with the Sprawling mutation were unable to sense the pain, but their other sensory functions were unaffected.[11]
Limitations
The tail flick test is one test to measure heat-induced pain in animals. This reflexive response is an indicator of pain sensitivity in an organism and reduction of pain sensitivity produced by analgesics. Limitations of this test include: the need for more research with murine subjects, and determining the validity of applying observed pain responses from animals to humans.[12] Also, researchers have found that skin temperature can significantly affect the results of the tail flick test and it is important to consider this effect when performing the test.[13] Lastly, many thermal tests do not distinguish between opioid agonists and mixed agonist-antagonists, and consequently a tail flick test for mice using cold water in place of heat has been developed to allow that distinction.[14]
References
- D'Amour, FE; Smith, DL (1941). "A method for determining loss of pain sensation". J Pharmacol Exp Ther. 72 (1): 74–78.
- Tzschentke, Thomas M.; Christoph, Thomas; Kögel, Babette; Schiene, Klaus; Hennies, Hagen-Heinrich; Englberger, Werner; Haurand, Michael; Jahnel, Ulrich; Cremers, Thomas I. F. H.; Friderichs, Elmar; De Vry, Jean (23 July 2007). "( )-(1R,2R)-3-(3-Dimethylamino-1-ethyl-2-methyl-propyl)-phenol Hydrochloride (Tapentadol HCl): a Novel -Opioid Receptor Agonist/Norepinephrine Reuptake Inhibitor with Broad-Spectrum Analgesic Properties". Journal of Pharmacology and Experimental Therapeutics. 323 (1): 265–276. doi:10.1124/jpet.107.126052. PMID 17656655. S2CID 942191.
- Mouse Tail-Flick, Allegheny College – via youtube.com.
- O'Dell, T; Wilson, L; Napoli, M; White, H; Mirsky, J. (1960). "Pharmacology of a Series of New 2-Substituted Pyridine Derivatives with Emphasis on their Analgesic and Interneuronal Blocking Properties". Journal of Pharmacology and Experimental Therapeutics. 128: 65–74. PMID 14428053.
- US Patent 3303199, Doebel, K & Gagneux, A., "Certain Imidazolone Derivatives and Process for Making Same", published 1967-02-07
- Irwin S, Houde RW, Bennett DR, Hendershot LC, Seevers MH (February 1951). "The effects of morphine methadone and meperidine on some reflex responses of spinal animals to nociceptive stimulation". J. Pharmacol. Exp. Ther. 101 (2): 132–43. PMID 14814606.
- Fender C, Fujinaga M, Maze M (January 2000). "Strain differences in the antinociceptive effect of nitrous oxide on the tail flick test in rats". Anesth. Analg. 90 (1): 195–9. doi:10.1097/00000539-200001000-00039. PMID 10625003. S2CID 36318349.
- Li, G.; Rhodes, J.; Girard, I.; Gammie, S.; Garland Jr., T. (2004). "Opioid-mediated pain sensitivity in mice bred for high voluntary wheel running". Physiology & Behavior. 83 (3): 515–524. doi:10.1016/j.physbeh.2004.09.003. PMID 15581674. S2CID 10496331.
- Rand, R; Burton, A; Ing, T (1965). "The Tail of the Rat, in Temperature Regulation and Acclimatization". Canadian Journal of Physiology and Pharmacology. 2012 (2): 257–267. doi:10.1139/y65-025. PMID 14329334.
- Mogil, Jeffrey S. (2007). "The Surprising Complexity of Pain Testing in the Laboratory Mouse". In Crawley, J (ed.). What's Wrong with My Mouse? Strategies for Rodent Behavior Phenotyping (PDF). San Diego, CA: Society for Neuroscience. p. 11-23.
- Chen, X.-J. (2007). "Proprioceptive Sensory Neuropathy in Mice with a Mutation in the Cytoplasmic Dynein Heavy Chain 1 Gene". Journal of Neuroscience. 27 (52): 14515–14524. doi:10.1523/JNEUROSCI.4338-07.2007. PMC 6673431. PMID 18160659.
- Le Bars, D; Gozariu, M; Cadden, S. (2001). "Animal Models of Nociception". Journal of Pharmacology and Experimental Therapeutics. 53 (4): 597–652. PMID 11734620.
- Berge OG, Garcia-Cabrera I, Hole K (April 1988). "Response latencies in the tail-flick test depend on tail skin temperature". Neurosci. Lett. 86 (3): 284–8. doi:10.1016/0304-3940(88)90497-1. PMID 3380319. S2CID 43231682.
- Pizziketti RJ, Pressman NS, Geller EB, Cowan A, Adler MW (December 1985). "Rat cold water tail-flick: a novel analgesic test that distinguishes opioid agonists from mixed agonist-antagonists". Eur. J. Pharmacol. 119 (1–2): 23–9. doi:10.1016/0014-2999(85)90317-6. PMID 2867920.