Thyroid's secretory capacity
Thyroid's secretory capacity (GT, also referred to as thyroid's incretory capacity, maximum thyroid hormone output, T4 output or, if calculated from serum levels of thyrotropin and thyroxine, as SPINA-GT[lower-alpha 1]) is the maximum stimulated amount of thyroxine that the thyroid can produce in a given time-unit (e.g. one second).[2][3]
| Thyroid's secretory capacity | |
|---|---|
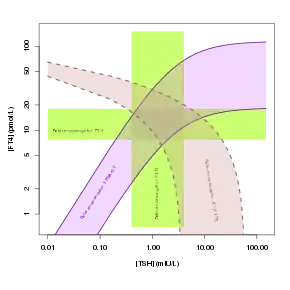 Reference ranges for SPINA-GT and other thyroid function tests | |
| Synonyms | SPINA-GT, GT, T4 output, thyroid hormone output, thyroid's incretory capacity, functional thyroid capacity[1] |
| Reference range | 1.41–8.67 pmol/s |
| Test of | Maximum amount of T4 produced by the thyroid in one second |
| MeSH | D013960 |
| LOINC | 82368-2 |
How to determine GT
Experimentally, GT can be determined by stimulating the thyroid with a high thyrotropin concentration (e.g. by means of rhTSH, i.e. recombinant human thyrotropin) and measuring its output in terms of T4 production, or by measuring the serum concentration of protein-bound iodine-131 after administration of radioiodine.[4] These approaches are, however, costly and accompanied by significant exposure to radiation.[5]
In vivo, GT can also be estimated from equilibrium levels of TSH and T4 or free T4. In this case it is calculated with
or
[TSH]: Serum thyrotropin concentration (in mIU/L or μIU/mL)
[FT4]: Serum free T4 concentration (in pmol/L)
[TT4]: Serum total T4 concentration (in nmol/L)
: Theoretical (apparent) secretory capacity (SPINA-GT)
: Dilution factor for T4 (reciprocal of apparent volume of distribution, 0.1 L−1)
: Clearance exponent for T4 (1.1e-6 sec−1), i. e., reaction rate constant for degradation
K41: Binding constant T4-TBG (2e10 L/mol)
K42: Binding constant T4-TBPA (2e8 L/mol)
DT: EC50 for TSH (2.75 mU/L)[2][6]
The method is based on mathematical models of thyroid homeostasis.[2][3] Calculating the secretory capacity with one of these equations is an inverse problem. Therefore, certain conditions (e.g. stationarity) have to be fulfilled to deliver a reliable result.
Specific secretory capacity
The ratio of SPINA-GT and thyroid volume VT (as determined e.g. by ultrasonography)
,
i.e.
or
is referred to as specific thyroid capacity (SPINA-GTs).[7] It is a measure for how much one millilitre of thyroid tissue can produce under conditions of maximum stimulation. Thereby, SPINA-GTs is an estimate for the endocrine quality of thyroid tissue.
Reference Range
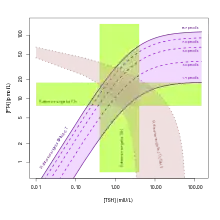
| Lower limit | Upper limit | Unit |
| 1.41[2] | 8.67[2] | pmol/s |
The equations and their parameters are calibrated for adult humans with a body mass of 70 kg and a plasma volume of ca. 2.5 L.[2]
Clinical significance
Validity
SPINA-GT is elevated in primary hyperthyroidism[8][9] and reduced in both primary hypothyroidism[10][11][12][9] and untreated autoimmune thyroiditis.[13] It has been observed to correlate (with positive direction) to resting energy expenditure,[14] resting heart rate,[15] the colour Doppler ultrasound pattern[16] and thyroid volume,[2][7] and (with negative direction) to thyroid autoantibody titres, which reflect organ destruction due to autoimmunity.[17] Elevated SPINA-GT in Graves' disease is reversible with antithyroid treatment.[14] While SPINA-GT is significantly altered in primary thyroid disorders, it is insensitive to disorders of secondary nature (e.g. pure pituitary diseases).[3]
Reliability
In silico experiments with Monte Carlo simulations demonstrated that both SPINA-GT and SPINA-GD can be estimated with sufficient reliability, even if laboratory assays have limited accuracy.[3] This was confirmed by longitudinal in vivo studies that showed that GT has lower intraindividual variation (i.e. higher reliability) than TSH, FT4 or FT3.[18]
Clinical utility
In clinical trials SPINA-GT was significantly elevated in patients with Graves' disease and toxic adenoma compared to normal subjects.[2][8] It is also elevated in diffuse and nodular goiters, and reduced in untreated autoimmune thyroiditis.[2][13] In patients with toxic adenoma it has higher specificity and positive likelihood ratio for diagnosis of thyrotoxicosis than serum concentrations of thyrotropin, free T4 or free T3.[2] GT's specificity is also high in thyroid disorders of secondary or tertiary origin.[3]
Calculating SPINA-GT has proved to be useful in challenging clinical situations, e.g. for differential diagnosis of subclinical hypothyroidism and elevated TSH concentration due to type 2 allostatic load (as it is typical for obesity and certain psychiatric diseases). For this purpose, its usage has been recommended in sociomedical assessment.[19]
Pathophysiological and therapeutic implications
Correlation of SPINA-GT with creatinine clearance suggests a negative influence of uremic toxins on thyroid biology.[20][21] In the initial phase of major non-thyroidal illness syndrome (NTIS) SPINA-GT may be temporarily elevated.[22][23] In chronic NTIS[24] as well as in certain non-critical chronic diseases, e.g. chronic fatigue syndrome[25][26] or asthma[27] SPINA-GT is slightly reduced.
According to the results of a community-based study in China it was associated to sleep duration and exercise habits.[28] With respect to iodine supply, it showed a complex U-shaped pattern, being reduced in subjects consuming iodine-rich food, but elevated in situations of iodine excess.[28] In two other studies from China, SPINA-GT correlated with negative direction to markers of obesity including body mass index, waist circumference and waist to hip ratio.[29][30] This doesn't seem to be the case, however, in Western populations.[31]
In women, therapy with Metformin results in increased SPINA-GT, in parallel to improved insulin sensitivity.[32] This observation was reproducible in men with hypogonadism, but not in men with normal testosterone concentrations,.[33] In postmenopausal women this effect was only observed in subjects on oestradiol replacement therapy.[34] Therefore, the described phenomenon seems to depend on an interaction of metformin with sex hormones.[33][35] In hyperthyroid[8] men both SPINA-GT and SPINA-GD negatively correlate to erectile function, intercourse satisfaction, orgasmic function and sexual desire. Likewise, in women with thyrotoxicosis elevated thyroid's secretory capacity predicts depression and sexual dysfunction.[36] Conversely, in androgen-deficient men with concomitant autoimmune thyroiditis, substitution therapy with testosterone leads to a decrease in thyroid autoantibody titres and an increase in SPINA-GT.[37]
In patients with autoimmune thyroiditis a gluten-free diet results in increased SPINA-GT (in parallel to sinking autoantibody titres).[38] Statin therapy has the same effect, but only if supply with vitamin D is sufficient.[39] Accordingly, substitution therapy with 25-hydroxyvitamin D leads to rising secretory capacity.[40][41][42][43] This effect is potentiated by substitution therapy with myo-inositol[44] and selenomethionine[40][41][45] or, in women, with dehydroepiandrosterone,[46] but impaired in males with early-onset androgenic alopecia.[47] The effects of vitamin D and selenomethionine are attenuated in hyperprolactinaemia, suggesting an inhibitory effect of prolactin.[48] Although both vitamin D supplementation and gluten-free diet result in increased SPINA-GT, there seems to be a complex interaction between both therapeutic measures, since vitamin D treatment is only able to elevate the thyroid's secretory capacity in subjects not following any dietary recommendation.[49]
On the other hand, men treated with spironolactone are faced with decreasing SPINA-GT (in addition to rising thyroid antibody titres).[50] It has, therefore, been concluded that spironolactone may aggravate thyroid autoimmunity in men.[50]
In subjects with type 2 diabetes, treatment with beta blockers resulted in decreased SPINA-GT, suggesting sympathetic innervation to contribute to the control of thyroid function.[51] In diabetic women, but not in men, SPINA-GT shows a positive correlation to the β-C-terminal cross-linked telopeptides of type I collagen (β-CTX), a marker of bone resorption.[52] In both diabetic and non-diabetic persons it correlates (negatively) with age and (positively) with the concentrations of troponin T and HbA1c.[53]
A study in euthyroid subjects with structural heart disease found that increased SPINA-GT predicts the risk of malignant arrhythmia including ventricular fibrillation and ventricular tachycardia.[54] This applies to both incidence and event-free survival.[54] Likewise, SPINA-GT is elevated in a significant subgroup of patients with takotsubo syndrome.[55] On the other hand, two studies found negative correlation between SPINA-GT and markers of dispersion in cardiac repolarisation, including Tp-e interval, JT interval, Tp-e/ QT ratio and Tp-e/QTc ratio. These results suggest that reduced thyroid function may trigger cardiovascular mortality as well.[56][9]
Among subjects with Parkinson's disease, SPINA-GT is significantly elevated in tremor-dominant and mixed subtypes compared to the akinetic-rigid type.[57]
Specific secretory capacity (SPINA-GTs) is reduced in obesity[2] and autoimmune thyroiditis.[7][58]
Endocrine disruptors may affect stimulated thyroid output, as demonstrated by a positive correlation of SPINA-GT with exposure to 2-hydroxynaphthalene (2-NAP),[59] urinary mercury concentration[60] and the excretion of certain phthalate metabolites,[61] and negative correlation with combined exposure to polycyclic aromatic hydrocarbons (PAHs)[59] and nickel.[62]
See also
Notes
- SPINA is an acronym for "structure parameter inference approach".
References
- Hoermann, R; Midgley, JEM; Larisch, R; Dietrich, JW (4 August 2021). "Treatment options for subclinical hypothyroidism". European Journal of Endocrinology. 185 (3): L5–L6. doi:10.1530/EJE-20-1405. PMID 34243143.
- Dietrich, J. W. (2002). Der Hypophysen-Schilddrüsen-Regelkreis. Berlin, Germany: Logos-Verlag Berlin. ISBN 978-3-89722-850-4. OCLC 50451543. OL 24586469M.
- Dietrich, Johannes W.; Landgrafe-Mende, Gabi; Wiora, Evelin; Chatzitomaris, Apostolos; Klein, Harald H.; Midgley, John E. M.; Hoermann, Rudolf (9 June 2016). "Calculated Parameters of Thyroid Homeostasis: Emerging Tools for Differential Diagnosis and Clinical Research". Frontiers in Endocrinology. 7: 57. doi:10.3389/fendo.2016.00057. PMC 4899439. PMID 27375554.
- Bierich, J. R. (1964). "Endokrinologie". In H. Wiesener (ed.). Einführung in die Entwicklungsphysiologie des Kindes. Springer. p. 310. ISBN 978-3-642-86507-7.
- Thompson, MA (June 2001). "Radiation safety precautions in the management of the hospitalized (131)I therapy patient". Journal of Nuclear Medicine Technology. 29 (2): 61–6, test 74–5. PMID 11376097.
- Dietrich JW, Stachon A, Antic B, Klein HH, Hering S (Oct 2008). "The AQUA-FONTIS study: protocol of a multidisciplinary, cross-sectional and prospective longitudinal study for developing standardized diagnostics and classification of non-thyroidal illness syndrome". BMC Endocrine Disorders. 8 (1): 13. doi:10.1186/1472-6823-8-13. PMC 2576461. PMID 18851740.
- Hoermann, Rudolf; Midgley, John E.M.; Larisch, Rolf; Dietrich, Johannes W. (18 August 2016). "Relational Stability of Thyroid Hormones in Euthyroid Subjects and Patients with Autoimmune Thyroid Disease". European Thyroid Journal. 5 (3): 171–179. doi:10.1159/000447967. PMC 5091265. PMID 27843807.
- Krysiak, R; Marek, B; Okopień, B (2019). "Sexual function and depressive symptoms in men with overt hyperthyroidism". Endokrynologia Polska. 70 (1): 64–71. doi:10.5603/EP.a2018.0069. PMID 30307028.
- Aweimer, Assem; Schiedat, Fabian; Schöne, Dominik; Landgrafe-Mende, Gabi; Bogossian, Harilaos; Mügge, Andreas; Patsalis, Polykarpos C.; Gotzmann, Michael; Akin, Ibrahim; El-Battrawy, Ibrahim; Dietrich, Johannes W. (23 November 2021). "Abnormal Cardiac Repolarization in Thyroid Diseases: Results of an Observational Study". Frontiers in Cardiovascular Medicine. 8: 738517. doi:10.3389/fcvm.2021.738517. PMC 8649843. PMID 34888359.
- Dietrich, J.; Fischer, M.; Jauch, J.; Pantke, E.; Gärtner, R.; Pickardt, C. R. "SPINA-THYR: A Novel Systems Theoretic Approach to Determine the Secretion Capacity of the Thyroid Gland". European Journal of Internal Medicine. 10 (Suppl. 1): S34.
- Dietrich JW (Sep 2012). "Thyroid storm". Medizinische Klinik, Intensivmedizin und Notfallmedizin. 107 (6): 448–53. doi:10.1007/s00063-012-0113-2. PMID 22878518. S2CID 31285541.
- Wang, X; Liu, H; Chen, J; Huang, Y; Li, L; Rampersad, S; Qu, S (21 April 2016). "Metabolic Characteristics in Obese Patients Complicated by Mild Thyroid Hormone Deficiency". Hormone and Metabolic Research. 48 (5): 331–7. doi:10.1055/s-0042-105150. PMID 27101096.
- Hoermann, R; Midgley, JEM; Larisch, R; Dietrich, JW (19 July 2018). "The Role of Functional Thyroid Capacity in Pituitary Thyroid Feedback Regulation". European Journal of Clinical Investigation. 48 (10): e13003. doi:10.1111/eci.13003. PMID 30022470. S2CID 51698223.
- Kim, Min Joo; Cho, Sun Wook; Choi, Sumin; Ju, Dal Lae; Park, Do Joon; Park, Young Joo (2018). "Changes in Body Compositions and Basal Metabolic Rates during Treatment of Graves' Disease". International Journal of Endocrinology. 2018: 9863050. doi:10.1155/2018/9863050. PMC 5960571. PMID 29853888.
- Steinberger, Eva; Pilz, Stefan; Trummer, Christian; Theiler-Schwetz, Verena; Reichhartinge, Markus; Benninger, Thomas; Pandis, Marlene; Malle, Oliver; Keppel, Martin H.; Verheyen, Nicolas; Grübler, Martin R.; Voelkl, Jakob; Meinitzer, Andreas; März, Winfried (4 September 2020). "Associations of Thyroid Hormones and Resting Heart Rate in Patients Referred to Coronary Angiography". Hormone and Metabolic Research. 52 (12): a–1232–7292. doi:10.1055/a-1232-7292. PMID 32886945. S2CID 221502851.
- Zhang, Lingyun; Li, Jie; Zhang, Suzhen; Su, Chen; Su, Zengcun; Zhang, Yuezhong; Gai, Yonghao; Shao, Shanshan; Li, Jianzhi; Zhang, Guoquan (30 July 2022). "Study of the Associations between Color Doppler Ultrasound Grading of Hyperthyroidism and Biochemical Data on Thyroid Function". International Journal of Endocrinology. 2022: 1–6. doi:10.1155/2022/9743654. PMC 9356896. PMID 35942151.
- Krysiak, R; Kowalcze, K; Okopień, B (20 May 2019). "The Effect of Selenomethionine on Thyroid Autoimmunity in Euthyroid Men With Hashimoto Thyroiditis and Testosterone Deficiency". Journal of Clinical Pharmacology. 59 (11): 1477–1484. doi:10.1002/jcph.1447. PMID 31106856. S2CID 159040151.
- Dietrich JW, Landgrafe G, Fotiadou EH (2012). "TSH and Thyrotropic Agonists: Key Actors in Thyroid Homeostasis". Journal of Thyroid Research. 2012: 351864. doi:10.1155/2012/351864. PMC 3544290. PMID 23365787.
- Dietrich, Johannes W.; Schifferdecker, Ekkehard; Schatz, Helmut; Klein, Harald (2022). "Endokrine und Stoffwechseldiagnostik". Die Ärztliche Begutachtung. Springer Reference Medizin. pp. 1–13. doi:10.1007/978-3-662-61937-7_83-1. ISBN 978-3-662-61937-7.
- Rosolowska-Huszcz D, Kozlowska L, Rydzewski A (Aug 2005). "Influence of low protein diet on nonthyroidal illness syndrome in chronic renal failure". Endocrine. 27 (3): 283–8. doi:10.1385/endo:27:3:283. PMID 16230785. S2CID 25630198.
- Yang, S; Lai, S; Wang, Z; Liu, A; Wang, W; Guan, H (December 2021). "Thyroid Feedback Quantile-based Index correlates strongly to renal function in euthyroid individuals". Annals of Medicine. 53 (1): 1945–1955. doi:10.1080/07853890.2021.1993324. PMC 8567884. PMID 34726096.
- Liu S, Ren J, Zhao Y, Han G, Hong Z, Yan D, Chen J, Gu G, Wang G, Wang X, Fan C, Li J (2013). "Nonthyroidal Illness Syndrome: is it Far Away From Crohn's Disease?". Journal of Clinical Gastroenterology. 47 (2): 153–9. doi:10.1097/MCG.0b013e318254ea8a. PMID 22874844. S2CID 35344744.
- Wan, S; Yang, J; Gao, X; Zhang, L; Wang, X (22 July 2020). "Nonthyroidal Illness Syndrome in Patients With Short-Bowel Syndrome". Journal of Parenteral and Enteral Nutrition. 45 (5): 973–981. doi:10.1002/jpen.1967. PMID 32697347. S2CID 220698496.
- Dietrich, J. W.; Ackermann, A.; Kasippillai, A.; Kanthasamy, Y.; Tharmalingam, T.; Urban, A.; Vasileva, S.; Schildhauer, T. A.; Klein, H. H.; Stachon, A.; Hering, S. (19 September 2019). "Adaptive Veränderungen des Schilddrüsenstoffwechsels als Risikoindikatoren bei Traumata". Trauma und Berufskrankheit. 21 (4): 260–267. doi:10.1007/s10039-019-00438-z. S2CID 202673793.
- Ruiz-Núñez, Begoña; Tarasse, Rabab; Vogelaar, Emar F.; Janneke Dijck-Brouwer, D. A.; Muskiet, Frits A. J. (20 March 2018). "Higher Prevalence of "Low T3 Syndrome" in Patients With Chronic Fatigue Syndrome: A Case–Control Study". Frontiers in Endocrinology. 9: 97. doi:10.3389/fendo.2018.00097. PMC 5869352. PMID 29615976.
- Sun, Q; Oltra, E; Dijck-Brouwer, DAJ; Chillon, TS; Seemann, P; Asaad, S; Demircan, K; Espejo-Oltra, JA; Sánchez-Fito, T; Martín-Martínez, E; Minich, WB; Muskiet, FAJ; Schomburg, L (3 July 2023). "Autoantibodies to selenoprotein P in chronic fatigue syndrome suggest selenium transport impairment and acquired resistance to thyroid hormone". Redox Biology. 65: 102796. doi:10.1016/j.redox.2023.102796. PMC 10338150. PMID 37423160.
- Bingyan, Zhan; Dong, Wei (7 July 2019). "Impact of thyroid hormones on asthma in older adults". Journal of International Medical Research. 47 (9): 4114–4125. doi:10.1177/0300060519856465. PMC 6753544. PMID 31280621.
- Wu, Kejun; Zhou, Yu; Ke, Sujie; Huang, Jingze; Gao, Xuelin; Li, Beibei; Lin, Xiaoying; Liu, Xiaohong; Liu, Xiaoying; Ma, Li; Wang, Linxi; Wu, Li; Wu, Lijuan; Xie, Chengwen; Xu, Junjun; Wang, Yanping; Liu, Libin (December 2021). "Lifestyle is associated with thyroid function in subclinical hypothyroidism: a cross-sectional study". BMC Endocrine Disorders. 21 (1): 112. doi:10.1186/s12902-021-00772-z. PMC 8161919. PMID 34049544.
- Zhou, Yu; Ke, Sujie; Wu, Kejun; Huang, Jingze; Gao, Xuelin; Li, Beibei; Lin, Xiaoying; Liu, Xiaohong; Liu, Xiaoying; Ma, Li; Wang, Linxi; Wu, Li; Wu, Lijuan; Xie, Chengwen; Xu, Junjun; Wang, Yanping; Liu, Libin (2021-05-29). "Correlation between Thyroid Homeostasis and Obesity in Subclinical Hypothyroidism: Community-Based Cross-Sectional Research". International Journal of Endocrinology. 2021: 1–7. doi:10.1155/2021/6663553. PMC 8179776. PMID 34135958.
- Yang, L; Sun, X; Tao, H; Zhao, Y (February 2023). "The association between thyroid homeostasis parameters and obesity in subjects with euthyroidism". Journal of Physiology and Pharmacology. 74 (1). doi:10.26402/jpp.2023.1.07. PMID 37245234.
- Ittermann, T; Markus, MRP; Bahls, M; Felix, SB; Steveling, A; Nauck, M; Völzke, H; Dörr, M (2021-05-18). "Low serum TSH levels are associated with low values of fat-free mass and body cell mass in the elderly". Scientific Reports. 11 (1): 10547. Bibcode:2021NatSR..1110547I. doi:10.1038/s41598-021-90178-7. PMC 8131378. PMID 34006958.
- Krysiak, R; Szkróbka, W; Okopień, B (June 2018). "Sex-Dependent Effect of Metformin on Serum Prolactin Levels In Hyperprolactinemic Patients With Type 2 Diabetes: A Pilot Study". Experimental and Clinical Endocrinology & Diabetes. 126 (6): 342–348. doi:10.1055/s-0043-122224. PMID 29169197. S2CID 43144838.
- Krysiak, R; Szkróbka, W; Okopień, B (6 August 2019). "The Impact of Testosterone on Metformin Action on Hypothalamic-Pituitary-Thyroid Axis Activity in Men: A Pilot Study". Journal of Clinical Pharmacology. 60 (2): 164–171. doi:10.1002/jcph.1507. PMID 31389032. S2CID 199466858.
- Krysiak, R; Kowalcze, K; Okopień, B (November 2021). "The impact of metformin on hypothalamic-pituitary-thyroid axis activity in postmenopausal women with untreated non-autoimmune subclinical hypothyroidism". Clinical and Experimental Pharmacology & Physiology. 48 (11): 1469–1476. doi:10.1111/1440-1681.13542. PMID 34145615. S2CID 235481699.
- Krysiak, Robert; Kowalcze, Karolina; Wolnowska, Monika; Okopień, Bogusław (5 January 2020). "The impact of oral hormonal contraception on metformin action on hypothalamic‐pituitary‐thyroid axis activity in women with diabetes and prediabetes: A pilot study". Journal of Clinical Pharmacy and Therapeutics. 45 (5): 937–945. doi:10.1111/jcpt.13105. PMID 31903641. S2CID 209895460.
- Krysiak, R; Kowalcze, K; Okopień, B (9 January 2019). "Sexual function and depressive symptoms in young women with overt hyperthyroidism". European Journal of Obstetrics, Gynecology, and Reproductive Biology. 234: 43–48. doi:10.1016/j.ejogrb.2018.12.035. PMID 30654201. S2CID 58558358.
- Krysiak, Robert; Kowalcze, Karolina; Okopień, Bogusław (10 June 2019). "The effect of testosterone on thyroid autoimmunity in euthyroid men with Hashimoto's thyroiditis and low testosterone levels". Journal of Clinical Pharmacy and Therapeutics. 44 (5): 742–749. doi:10.1111/jcpt.12987. PMID 31183891. S2CID 184487697.
- Krysiak, R; Szkróbka, W; Okopień, B (30 July 2018). "The Effect of Gluten-Free Diet on Thyroid Autoimmunity in Drug-Naïve Women with Hashimoto's Thyroiditis: A Pilot Study". Experimental and Clinical Endocrinology & Diabetes. 127 (7): 417–422. doi:10.1055/a-0653-7108. PMID 30060266. S2CID 51874521.
- Krysiak, R; Szkróbka, W; Okopień, B (27 August 2018). "The Relationship Between Statin Action On Thyroid Autoimmunity And Vitamin D Status: A Pilot Study". Experimental and Clinical Endocrinology & Diabetes. 127 (1): 23–28. doi:10.1055/a-0669-9309. PMID 30149415. S2CID 52100009.
- Krysiak, Robert; Szkróbka, Witold; Okopień, Bogusław (October 2018). "The effect of vitamin D and selenomethionine on thyroid antibody titers, hypothalamic-pituitary-thyroid axis activity and thyroid function tests in men with Hashimoto's thyroiditis: a pilot study". Pharmacological Reports. 71 (2): 243–7. doi:10.1016/j.pharep.2018.10.012. PMID 30818086. S2CID 73481267.
- Krysiak, Robert; Kowalcze, Karolina; Okopień, Bogusław (December 2018). "Selenomethionine potentiates the impact of vitamin D on thyroid autoimmunity in euthyroid women with Hashimoto's thyroiditis and low vitamin D status". Pharmacological Reports. 71 (2): 367–73. doi:10.1016/j.pharep.2018.12.006. PMID 30844687. S2CID 73486105.
- Krysiak, Robert; Kowalcze, Karolina; Okopień, Bogusław (April 2019). "The effect of vitamin D on thyroid autoimmunity in euthyroid men with autoimmune thyroiditis and testosterone deficiency". Pharmacological Reports. 71 (5): 798–803. doi:10.1016/j.pharep.2019.04.010. PMID 31377561.
- Krysiak, Robert; Kowalcze, Karolina; Szkróbka, Witold; Okopień, Bogusław (20 June 2023). "Sexual Function and Depressive Symptoms in Young Women with Euthyroid Hashimoto's Thyroiditis Receiving Vitamin D, Selenomethionine and Myo-Inositol: A Pilot Study". Nutrients. 15 (12): 2815. doi:10.3390/nu15122815. PMC 10304218. PMID 37375719.
- Krysiak, R; Kowalcze, K; Okopień, B (30 June 2022). "The impact of vitamin D on thyroid autoimmunity and hypothalamic-pituitary-thyroid axis activity in myo-inositol-treated and myo-inositol-naïve women with autoimmune thyroiditis: A pilot study". Journal of Clinical Pharmacy and Therapeutics. 47 (11): 1759–1767. doi:10.1111/jcpt.13730. PMID 35775148. S2CID 250174449.
- Saini, Chandanvir (6 April 2021). "Autoimmune thyroiditis and multiple nutritional factors". International Journal of Endocrinology (Ukraine). 16 (8): 648–653. doi:10.22141/2224-0721.16.8.2020.222885.
- Krysiak, R; Szkróbka, W; Okopień, B (February 2021). "Dehydroepiandrosterone potentiates the effect of vitamin D on thyroid autoimmunity in euthyroid women with autoimmune thyroiditis: A pilot study". Clinical and Experimental Pharmacology & Physiology. 48 (2): 195–202. doi:10.1111/1440-1681.13410. PMID 33007106. S2CID 222165571.
- Krysiak, R; Kowalcze, K; Okopień, B (2021-06-09). "The impact of exogenous vitamin D on thyroid autoimmunity in euthyroid men with autoimmune thyroiditis and early-onset androgenic alopecia". Pharmacological Reports. 73 (5): 1439–1447. doi:10.1007/s43440-021-00295-3. PMC 8460519. PMID 34106452.
- Krysiak, R; Kowalcze, K; Okopień, B (10 July 2020). "Hyperprolactinaemia attenuates the inhibitory effect of vitamin D/selenomethionine combination therapy on thyroid autoimmunity in euthyroid women with Hashimoto's thyroiditis: A pilot study". Journal of Clinical Pharmacy and Therapeutics. 45 (6): 1334–1341. doi:10.1111/jcpt.13214. PMID 32649802. S2CID 220485158.
- Krysiak, R; Kowalcze, K; Okopień, B (6 October 2022). "Gluten-free diet attenuates the impact of exogenous vitamin D on thyroid autoimmunity in young women with autoimmune thyroiditis: a pilot study". Scandinavian Journal of Clinical and Laboratory Investigation. 82 (7–8): 518–524. doi:10.1080/00365513.2022.2129434. PMID 36200764. S2CID 252736895.
- Krysiak, R; Kowalcze, K; Okopień, B (14 September 2019). "The effect of spironolactone on thyroid autoimmunity in euthyroid men with Hashimoto's thyroiditis". Journal of Clinical Pharmacy and Therapeutics. 45 (1): 152–159. doi:10.1111/jcpt.13046. PMID 31520539.
- Yang, Lijuan; Sun, Xiuqin; Zhao, Yi; Tao, Hong (7 March 2022). "Effects of Antihypertensive Drugs on Thyroid Function in Type 2 Diabetes Patients With Euthyroidism". Frontiers in Pharmacology. 13: 802159. doi:10.3389/fphar.2022.802159. PMC 8940167. PMID 35330837.
- Chen, Y; Zhang, W; Chen, C; Wang, Y; Wang, N; Lu, Y (31 March 2022). "Thyroid and bone turnover markers in type 2 diabetes: results from the METAL study". Endocrine Connections. 11 (3). doi:10.1530/EC-21-0484. PMC 9010813. PMID 35196256.
- Li, W; He, Q; Zhang, H; Shu, S; Wang, L; Wu, Y; Yuan, Z; Zhou, J (20 January 2023). "Thyroid-stimulating hormone within the normal reference range has a U-shaped association with the severity of coronary artery disease in nondiabetic patients but is diluted in diabetic patients". Journal of Investigative Medicine. 71 (4): 350–360. doi:10.1177/10815589221149187. PMID 36680358. S2CID 256055662.
- Müller, Patrick; Dietrich, Johannes W.; Lin, Tina; Bejinariu, Alexandru; Binnebößel, Stephan; Bergen, Friederike; Schmidt, Jan; Müller, Sarah-Kristin; Chatzitomaris, Apostolos; Kurt, Muhammed; Gerguri, Shqipe; Clasen, Lukas; Klein, Harald H.; Kelm, Malte; Makimoto, Hisaki (January 2020). "Usefulness of Serum Free Thyroxine Concentration to Predict Ventricular Arrhythmia Risk in Euthyroid Patients with Structural Heart Disease". The American Journal of Cardiology. 125 (8): 1162–1169. doi:10.1016/j.amjcard.2020.01.019. PMID 32087999. S2CID 211261823.
- Aweimer, A; El-Battrawy, I; Akin, I; Borggrefe, M; Mügge, A; Patsalis, PC; Urban, A; Kummer, M; Vasileva, S; Stachon, A; Hering, S; Dietrich, JW (12 November 2020). "Abnormal thyroid function is common in takotsubo syndrome and depends on two distinct mechanisms: results of a multicentre observational study". Journal of Internal Medicine. 289 (5): 675–687. doi:10.1111/joim.13189. PMID 33179374.
- Gürdal, A; Eroğlu, H; Helvaci, F; Sümerkan, MÇ; Kasali, K; Çetin, Ş; Aksan, G; Kiliçkesmez, K (March 2017). "Evaluation of Tp-e interval, Tp-e/QT ratio and Tp-e/QTc ratio in patients with subclinical hypothyroidism". Therapeutic Advances in Endocrinology and Metabolism. 8 (3): 25–32. doi:10.1177/2042018816684423. PMC 5363453. PMID 28377800.
- Tan, Y; Gao, L; Yin, Q; Sun, Z; Man, X; Du, Y; Chen, Y (April 2021). "Thyroid hormone levels and structural parameters of thyroid homeostasis are correlated with motor subtype and disease severity in euthyroid patients with Parkinson's disease". The International Journal of Neuroscience. 131 (4): 346–356. doi:10.1080/00207454.2020.1744595. PMID 32186220. S2CID 212752563.
- Hoermann, Rudolf; Midgley, John E. M.; Larisch, Rolf; Dietrich, Johannes W. (7 November 2016). "Relational Stability in the Expression of Normality, Variation, and Control of Thyroid Function". Frontiers in Endocrinology. 7: 142. doi:10.3389/fendo.2016.00142. PMC 5098235. PMID 27872610.
- Yang, S; Sun, J; Wang, S; E, L; Zhang, S; Jiang, X (9 August 2023). "Association of exposure to polycyclic aromatic hydrocarbons with thyroid hormones in adolescents and adults, and the influence of the iodine status". Environmental Science. Processes & Impacts. 25 (9): 1449–1463. doi:10.1039/d3em00135k. PMID 37555279. S2CID 259916981.
- Kim, Min Joo; Kim, Sunmi; Choi, Sohyeon; Lee, Inae; Moon, Min Kyong; Choi, Kyungho; Park, Young Joo; Cho, Yoon Hee; Kwon, Young Min; Yoo, Jiyoung; Cheon, Gi Jeong; Park, Jeongim (December 2020). "Association of exposure to polycyclic aromatic hydrocarbons and heavy metals with thyroid hormones in general adult population and potential mechanisms". Science of the Total Environment. 762: 144227. doi:10.1016/j.scitotenv.2020.144227. PMID 33373756. S2CID 229722026.
- Chen, Y; Zhang, W; Chen, J; Wang, N; Chen, C; Wang, Y; Wan, H; Chen, B; Lu, Y (2021). "Association of Phthalate Exposure with Thyroid Function and Thyroid Homeostasis Parameters in Type 2 Diabetes". Journal of Diabetes Research. 2021: 4027380. doi:10.1155/2021/4027380. PMC 8566079. PMID 34746318.
- Maric, D; Baralic, K; Javorac, D; Mandic-Rajcevic, S; Zarkovic, M; Antonijevic, B; Djukic-Cosic, D; Bulat, Z; Djordjevic, AB (2023). "Nickel as a potential disruptor of thyroid function: benchmark modelling of human data". Frontiers in Endocrinology. 14: 1145153. doi:10.3389/fendo.2023.1145153. PMC 10549921. PMID 37800147.
External links
| Library resources about Thyroid's secretory capacity |
- SPINA Thyr: Open source software for calculating GT and GD
- Package "SPINA" for the statistical environment R