Trifluoroacetone
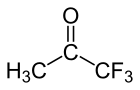
Trifluoroacetone (1,1,1-trifluoroacetone) is an organofluorine compound with the chemical formula CF3C(O)CH3.[1] The compound is a colorless liquid with chloroform-like odour.[2]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1,1,1-Trifluoropropan-2-one | |
| Other names
Trifluoracetone, TFA | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.006.370 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H3F3O | |
| Molar mass | 112.051 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.252 g/mL |
| Melting point | −78 °C (−108 °F; 195 K) |
| Boiling point | 21–24 °C (70–75 °F; 294–297 K) |
| Hazards | |
| GHS labelling: | |
  | |
| Danger | |
| H224, H315, H319, H335 | |
| P210, P261, P303, P338, P351 | |
| Flash point | −30 °C (−22 °F; 243 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Preparation, reactions, uses
Trifluoroacetone is produced from trifluoroacetoacetic acid, which is generated by condensation of ethyl trifluoroacete and ethyl acetate:
- CF3CO2C2H5 + CH3CO2C2H5 → CF3C(O)CH2CO2C2H5 + C2H5OH
Hydrolysis of the keto-ester affords trifluoroacetone:
- CF3C(O)CH2CO2C2H5 + H2O → CF3C(O)CH2CO2H + C2H5OH
- CF3C(O)CH2CO2H → CF3C(O)CH3 + CO2
Alternatively, addition of methylmagnesium iodide with trifluoroacetic acid gives the ketone according to this idealized equation:[2]
- CF3CO2H + 2 CH3MgI → CF3C(O)CH3 + MgI2 + CH4 + MgO
Reactions
Many studies report on the reactions of trifluoroacetone.[3] It is less prone to hydrate than hexafluoroacetone and more electrophilic than acetone itself. Unlike both of those ketones, trifluoroacetone is prochiral.
Hydrogenation of trifluoroacetone over platinum catalyst gives trifluoroisopropanol. The reduction can also be achieved asymmetrically. Similarly, alkylation with Grignard reagents provides a route to tertiary alcohols. Alkylation and arylation can be achieved using malonate anions and arenes/AlCl3, respectively.
Trifluoroacetone has been converted to the dioxirane using oxone.
It serves as an oxidizing agent in Oppenauer oxidation.[4]
Trifluoracetone is also used in a synthesis of 2-trifluoromethyl-7-azaindoles starting with 2,6-dihalopyridines. The derived chiral imine is used to prepare enantiopure α-trifluoromethyl alanines and diamines by a Strecker reaction followed by either nitrile hydrolysis or reduction.[5]
See also
References
- "1,1,1-Trifluoracetone 95%". dk.vwr.com. Retrieved 6 June 2017.
- Günter Siegemund; Werner Schwertfeger; Andrew Feiring; Bruce Smart; Fred Behr; Herward Vogel; Blaine McKusick (2002). "Fluorine Compounds, Organic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_349. ISBN 978-3-527-30673-2.
- Prakash, G. K.Surya; Wang, Fang (2011). "1,1,1-Trifluoroacetone". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rn01348. ISBN 978-0-471-93623-7.
- Mello, Rossella; Martínez-Ferrer, Jaime; Asensio, Gregorio; González-Núñez, María Elena (2007). "Oppenauer Oxidation of Secondary Alcohols with 1,1,1-Trifluoroacetone as Hydride Acceptor". J. Org. Chem. 24 (72): 9376–9378. doi:10.1021/jo7016422. PMID 17975928.
- "Concise synthesis of enantiopure alpha-trifluoromethyl alanines, diamines, and amino alcohols by the Strecker-type reaction". sigmaaldrich.com. Retrieved 6 June 2017.