Magnesium citrate (3:2)
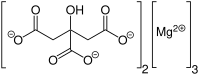
Magnesium citrate (3:2) (3 magnesium atoms per 2 citrate molecules), also called trimagnesium dicitrate, trimagnesium citrate, or the ambiguous name magnesium citrate. The substance magnesium citrate usually has water molecules attached to it. It is a (hydrated) salt of magnesium and citric acid. It is a bitter salt and dissolves with difficulty in water. It contains 16.2% elemental magnesium by weight. However, it can naturally only be available as nonahydrate (with 9 molecules of water to every molecule of trimagnesium dicitrate). This hydrated form only contains 12% elemental magnesium by weight.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Trimagnesium bis(2-hydroxypropane-1,2,3-tricarboxylate) | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.020.086 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C12H10Mg3O14 | |
| Molar mass | 451.113 g·mol−1 |
| Appearance | White powder |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.