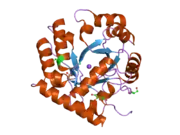Uridine monophosphate synthase
The enzyme Uridine monophosphate synthase (EC 4.1.1.23, UMPS) (orotate phosphoribosyl transferase and orotidine-5'-decarboxylase) catalyses the formation of uridine monophosphate (UMP), an energy-carrying molecule in many important biosynthetic pathways.[5] In humans, the gene that codes for this enzyme is located on the long arm of chromosome 3 (3q13).[6]
Structure and function
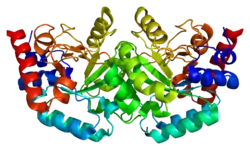
This bifunctional enzyme has two main domains, an orotate phosphoribosyltransferase (OPRTase, EC 2.4.2.10) subunit and an orotidine-5’-phosphate decarboxylase (ODCase, EC 4.1.1.23) subunit.[7] These two sites catalyze the last two steps of the de novo uridine monophosphate (UMP) biosynthetic pathway. After addition of ribose-P to orotate by OPRTase to form orotidine-5’-monophosphate (OMP), OMP is decarboxylated to form uridine monophosphate by ODCase. In microorganisms, these two domains are separate proteins, but, in multicellular eukaryotes, the two catalytic sites are expressed on a single protein, uridine monophosphate synthase.[8]
UMPS exists in various forms, depending on external conditions. In vitro, monomeric UMPS, with a sedimentation coefficient S20,w of 3.6 will become a dimer, S20,w = 5.1 after addition of anions such as phosphate. In the presence of OMP, the product of the OPRTase, the dimer changes to a faster-sedimenting form S20,w 5.6.[9][10] These separate conformational forms display different enzymatic activities, with the UMP synthase monomer displaying low decarboxylase activity, and only the 5.6 S dimer exhibiting full decarboxylase activity.[11]
It is believed that the two separate catalytic sites fused into a single protein to stabilize its monomeric form. The covalent union in UMPS stabilizes the domains containing the respective catalytic centers, improving its activity in multicellular organisms where concentrations tend to be 1/10th of the separate counterparts in prokaryotes. Other microorganisms with separated enzymes must retain higher concentrations to keep their enzymes in their more active dimeric form.[12]
Fusion
Fusion events between OPRTase and ODCase, which have led to the formation of the bifunctional enzyme UMPS, have occurred distinctly in different branches of the tree of life. For one thing, even though OPRTase is found at the N-terminus and ODCase at the C-terminus in most eukaryotes (e.g., Metazoa, Amoebozoa, Plantae, and Heterolobosea), the inverted fusion, which is to say OPRTase at the C-terminus and ODCase at the N-terminus, has also been shown to exist (e.g., parasitic protists, trypanosomatids, and stramenopiles). Moreover, other eukaryotic groups, such as Fungi, conserve both enzymes as separate proteins.[13]
However important the fusion order is, the evolutionary origin of each catalytic domain in UMPS is also a matter of study. Both OPRTase and ODCase have passed through lateral gene transfer, resulting in eukaryotes' having enzymes from bacterial and eukaryotic origin. For instance, Metazoa, Amoebozoa, Plantae, and Heterolobosea have eukaryotic ODCase and OPRTase, whereas Alveolata and stramenopiles have bacterial ones. Other rearrangements are also possible, since Fungi have bacterial OPRTase and eukaryotic ODCase, whereas kinetoplastids have the inverse combination.[13]
Merging both the fusion order and evolutionary origin, organisms end up having fused UMPS where one of its catalytic domains comes from bacteria and the other from eukaryotes.[13]
The driving force for these fusion events seems to be the acquired thermal stability. Homo sapiens OPRTase and ODCase activities lower to a greater extent when heated than the fused protein does.[14]
To determine the driving force of protein association, several experiments have been performed separating both domains and changing the linker peptide that keeps them together. In Plasmodium falciparum, the OPRTase-OMPDCase complex increases the kinetic and thermal stability when compared to monofunctional enzymes.[15] In H. sapiens, even though separate and fused domains have a similar activity, the former have a higher sensitivity to conditions promoting monomer dissociation.[12] Also, the linker peptide can be removed without inactivating catalysis.[14] In Leishmania donovani, separate OPRTase does not have detectable activity possibly due to lower thermal stability or lack of its linker peptide.[16]
Regulation
UMPS is subject to complex regulation by OMP, the product of its OPRTase and the substrate for the ODCase.[17] OMP is an allosteric activator of OMP decarboxylase activity.[10] At low enzyme concentration and low OMP concentrations, OMP decarboxylase shows negative cooperativity, whereas, at higher OMP concentrations, the enzyme shows positive cooperativity. However, when enzyme concentrations are higher, these complex kinetics do not manifest.[17] Orotate PRTase activity is activated by low concentrations of OMP,[18] phosphate,[8] and ADP.[19]
Mechanism
OPRTase
P. falciparum OPRTase follows a random pathway in OMP synthesis and degradation. Transition state analyses have used isotopic effects and quantum calculations to reveal a completely dissociated dianionic orotate structure, a ribocation, and a nucleophilic pyrophosphate molecule. Nonetheless, this is unexpected, since most N-ribosyltransferases involve protonated and neutral leaving groups, whereas deprotonated orotate is not a good one in the cationic transition state.[20]
OPRTase, as a member of type I PRTases, has a prominent loop next to its active site. It is flexible in its open state and can hardly be seen in electronic density maps for some OPRTases. For catalysis to occur, a dimer must exist in which a loop from one subunit covers the active site from the other one. In Salmonella typhimurium, a new pair of antiparallel β-sheets is created and five new interatomic contacts are formed in the loop, between the loop and the rest of the protein and between the loop and the ligands.[21]
There are two possibilities as far as the loop movement is concerned: It could move in a rigid manner or it could come from a disordered structure that acquires order. The second scenario seems more likely to occur in OPRTase. There must be an energy balance between the peptide new order and hydrogen bond formation in the loop, between the loop and the rest of the protein, and between the loop and the ligands. There is a 30:1 equilibrium between the close and open structures in the enzyme-Mg-PRPP complex, which suggests that the close conformation is favored.[21]
Various roles have been proposed to the catalytic loop residues. First of all, there seems to be a correlation between the loop movement and the substrate catalysis positioning. In the biological reaction, a proton transference to the pyrophosphate (PPi) molecule could minimize negative charge accumulation even though the pKa for PPi is 9. Lys26, His105, and Lys103 are candidates for this transference to the α phosphate position. However, it might not be the case, since lateral chains and the metal ion could neutralize some of the negative charge from the produced PPi. Transition-state geometric stabilization could also be gained through loop participation.[21]
ODCase
Callahan & Miller (2007) summarize ODCase mechanisms in three proposals. The first one is the substrate carboxyl activation through electrostatic stress. The phosphoryl group binding entails juxtaposition between the carboxylate group and a negatively charged Asp residue (namely Asp91 in Saccharomyces cerevisiae). Repulsion between the negative charges would raise the energy value near the transition state. Nonetheless, crystallographic analyses and the lack of S. cerevisiae enzyme affinity to substrate analogues where the carboxylate groups is replaced by a cationic substituent have shown some evidence against this theory.[22]
OMP protonation on O4 or O2 before decarboxylation, which entails and ylide formation on N1, has also been considered. Proton donor absence near O4 or O2 in crystallographic structures is evidence against it along with the ylide generation exclusion as a limiting step in 15N experiments. Moreover, doubts have aroused as to protonated intermediate viability due to electronic stabilizers absence. As a consequence, bond rupture between C6 and C7 due to protonation of the former going through a carbanion state has been proposed.[22]
Finally, catalysis might take place by simple electrostatic attraction. C6 carbanion formation would create dipole interactions with a cationic Lys from the active site. This does not explain the velocity increase when compared with the uncatalyzed process.[22]
Clinical significance
A UMP synthase deficiency can result in a metabolic disorder called orotic aciduria.[23]
Deficiency of this enzyme is an inherited autosomal recessive trait in Holstein cattle, and it will cause death before birth.[24]
Deficiency of the enzyme can be studied in the model organism Caenorhabditis elegans. The rad-6 strain has a premature stop codon eliminating the orotidine 5’-decarboxylase domain of the protein; this domain does not occur in any other proteins encoded by the genome. The strain has a pleiotropic phenotype including reduced viability and fertility, slow growth, and radiation sensitivity.[25]
Pharmacological importance
UMPS and its two separate domains, ODCase and OPRTase, have been shown to be essential to viability in parasites from the Chromoalveolata taxon such as L. donovani or P. falciparum.[16][26] Since UMPS, ODCase and OPRTase are different between organisms, research on species-specific inhibitors has been performed.[20][26]
Inhibition
OPRTase
Studies on OPRTase inhibition are based on substrate analogues. In Mycobacterium tuberculosis, two of the most promising inhibitors are 2,6-dihydroxipyridine-4-carboxylic acid and 3-benzylidene-2,6-dioxo-1,2,3,6-tetrahydropyridine-4-carboxylic acid. Union enthalpy and enthropy from the latter correspond to high-affinity ligands. Properties such as lipophilicity, solubility, permeability, and equilibrium constants are under study.[27]
Selenilation products have also been used. Abdo et al. (2010) performed reactions on 2-ethoxiethanselenic acid using electron-rich aromatic substrates to produce (2-ethoxiethyl)seleno ethers. These are able to become aryl-selenilated products such as the 5-uridinyl family, which has shown inhibition at submicromolar concentrations in P. falciparum and H. sapiens.[28]
ODCase
ODCase inhibitors also come from substrate analogues such as modifications on the OMP or UMP rings. In H. sapiens, ODCase has been inhibited by halide compounds derived from UMP (e.g., 5-FUMP, 5-BrUMP, 5-IUMP, and 6-IUMP.)[29]
In Methanobacterium thermoautotrophicum, a different strategy has been applied, modifying weak interacting ligands as cytidine-5’-monophosphate, which derivates into barbiturate ribonucleoside-5’-monophosphate, xantosine-5’-monophosphate.[30] P. falciparum ODCase has been successfully inhibited by modifications on cytidine-5’-monophosphate N3 and N4.[31]
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles.[§ 1]
- The interactive pathway map can be edited at WikiPathways: "FluoropyrimidineActivity_WP1601".
References
- GRCh38: Ensembl release 89: ENSG00000114491 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000022814 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Entrez Gene: UMPS uridine monophosphate synthase (orotate phosphoribosyl transferase and orotidine-5'-decarboxylase)".
- Qumsiyeh MB, Valentine MB, Suttle DP (Jul 1989). "Localization of the gene for uridine monophosphate synthase to human chromosome region 3q13 by in situ hybridization". Genomics. 5 (1): 160–2. doi:10.1016/0888-7543(89)90103-1. PMID 2767686.
- Traut TW, Jones ME (1996). Uracil metabolism--UMP synthesis from orotic acid or uridine and conversion of uracil to beta-alanine: enzymes and cDNAs. pp. 1–78. doi:10.1016/s0079-6603(08)60142-7. ISBN 9780125400534. PMID 8650301.
{{cite book}}:|journal=ignored (help) - Jones ME (1980). "Pyrimidine nucleotide biosynthesis in animals: genes, enzymes, and regulation of UMP biosynthesis". Annual Review of Biochemistry. 49: 253–79. doi:10.1146/annurev.bi.49.070180.001345. PMID 6105839.
- Traut TW, Jones ME (Feb 1979). "Interconversion of different molecular weight forms of the orotate phosphoribosyltransferase.orotidine-5'-phosphate decarboxylase enzyme complex from mouse Ehrlich ascites cells". The Journal of Biological Chemistry. 254 (4): 1143–50. doi:10.1016/S0021-9258(17)34180-7. PMID 762120.
- Traut TW, Payne RC, Jones ME (Dec 1980). "Dependence of the aggregation and conformation states of uridine 5'-phosphate synthase on pyrimidine nucleotides. Evidence for a regulatory site". Biochemistry. 19 (26): 6062–8. doi:10.1021/bi00567a018. PMID 6894093.
- Traut TW, Payne RC (Dec 1980). "Dependence of the catalytic activities on the aggregation and conformation states of uridine 5'-phosphate synthase". Biochemistry. 19 (26): 6068–74. doi:10.1021/bi00567a019. PMID 6894094.
- Yablonski MJ, Pasek DA, Han BD, Jones ME, Traut TW (May 1996). "Intrinsic activity and stability of bifunctional human UMP synthase and its two separate catalytic domains, orotate phosphoribosyltransferase and orotidine-5'-phosphate decarboxylase". The Journal of Biological Chemistry. 271 (18): 10704–8. doi:10.1074/jbc.271.18.10704. PMID 8631878.
- Makiuchi T, Nara T, Annoura T, Hashimoto T, Aoki T (Jun 2007). "Occurrence of multiple, independent gene fusion events for the fifth and sixth enzymes of pyrimidine biosynthesis in different eukaryotic groups". Gene. 394 (1–2): 78–86. doi:10.1016/j.gene.2007.02.009. PMID 17383832.
- Lin T, Suttle DP (May 1995). "UMP synthase activity expressed in deficient hamster cells by separate transferase and decarboxylase proteins or by linker-deleted bifunctional protein". Somatic Cell and Molecular Genetics. 21 (3): 161–75. doi:10.1007/bf02254768. PMID 7482031. S2CID 21932938.
- Kanchanaphum P, Krungkrai J (Dec 2009). "Kinetic benefits and thermal stability of orotate phosphoribosyltransferase and orotidine 5'-monophosphate decarboxylase enzyme complex in human malaria parasite Plasmodium falciparum". Biochemical and Biophysical Research Communications. 390 (2): 337–41. doi:10.1016/j.bbrc.2009.09.128. PMID 19800871.
- French JB, Yates PA, Soysa DR, Boitz JM, Carter NS, Chang B, Ullman B, Ealick SE (Jun 2011). "The Leishmania donovani UMP synthase is essential for promastigote viability and has an unusual tetrameric structure that exhibits substrate-controlled oligomerization". The Journal of Biological Chemistry. 286 (23): 20930–41. doi:10.1074/jbc.m111.228213. PMC 3121495. PMID 21507942.
- Traut TW (Jan 1989). "Uridine-5'-phosphate synthase: evidence for substrate cycling involving this bifunctional protein". Archives of Biochemistry and Biophysics. 268 (1): 108–15. doi:10.1016/0003-9861(89)90570-5. PMID 2912371.
- Traut TW, Jones ME (Dec 1977). "Kinetic and conformational studies of the orotate phosphoribosyltransferase:orotidine-5'-phosphate decarboxylase enzyme complex from mouse Ehrlich ascites cells". The Journal of Biological Chemistry. 252 (23): 8372–81. doi:10.1016/S0021-9258(19)75229-6. PMID 925000.
- Chen JJ, Jones ME (Apr 1979). "Effect of 5-phosphoribosyl-a-pyrophosphate on de novo pyrimidine biosynthesis in cultured Ehrlich ascites cells made permeable with dextran sulfate 500". The Journal of Biological Chemistry. 254 (8): 2697–704. doi:10.1016/S0021-9258(17)30128-X. PMID 218951.
- Zhang Y, Deng H, Schramm VL (Dec 2010). "Leaving group activation and pyrophosphate ionic state at the catalytic site of Plasmodium falciparum orotate phosphoribosyltransferase". Journal of the American Chemical Society. 132 (47): 17023–31. doi:10.1021/ja107806j. PMC 3012390. PMID 21067187.
- Wang GP, Hansen MR, Grubmeyer C (Jun 2012). "Loop residues and catalysis in OMP synthase". Biochemistry. 51 (22): 4406–15. doi:10.1021/bi300082s. PMC 3436960. PMID 22531099.
- Callahan BP, Miller BG (Dec 2007). "OMP decarboxylase--An enigma persists". Bioorganic Chemistry. 35 (6): 465–9. doi:10.1016/j.bioorg.2007.07.004. PMID 17889251.
- Suchi M, Mizuno H, Kawai Y, Tsuboi T, Sumi S, Okajima K, Hodgson ME, Ogawa H, Wada Y (Mar 1997). "Molecular cloning of the human UMP synthase gene and characterization of point mutations in two hereditary orotic aciduria families". American Journal of Human Genetics. 60 (3): 525–39. PMC 1712531. PMID 9042911.
- Shanks RD, Robinson JL (Nov 1989). "Embryonic mortality attributed to inherited deficiency of uridine monophosphate synthase". Journal of Dairy Science. 72 (11): 3035–9. doi:10.3168/jds.S0022-0302(89)79456-X. PMID 2625493.
- Merry A (2007). Characterisation and Identification of a Radiation Sensitive Mutant in Caenorhabditis elegans (Ph.D.). University of Bristol.
- Krungkrai SR, Aoki S, Palacpac NM, Sato D, Mitamura T, Krungkrai J, Horii T (Apr 2004). "Human malaria parasite orotate phosphoribosyltransferase: functional expression, characterization of kinetic reaction mechanism and inhibition profile". Molecular and Biochemical Parasitology. 134 (2): 245–55. doi:10.1016/j.molbiopara.2003.12.006. PMID 15003844.
- Breda A, Machado P, Rosado LA, Souto AA, Santos DS, Basso LA (Aug 2012). "Pyrimidin-2(1H)-ones based inhibitors of Mycobacterium tuberculosis orotate phosphoribosyltransferase". European Journal of Medicinal Chemistry. 54: 113–22. doi:10.1016/j.ejmech.2012.04.031. PMID 22608674.
- Abdo M, Zhang Y, Schramm VL, Knapp S (Jul 2010). "Electrophilic aromatic selenylation: new OPRT inhibitors". Organic Letters. 12 (13): 2982–5. doi:10.1021/ol1010032. PMC 2906230. PMID 20521773.
- Wittmann JG, Heinrich D, Gasow K, Frey A, Diederichsen U, Rudolph MG (Jan 2008). "Structures of the human orotidine-5'-monophosphate decarboxylase support a covalent mechanism and provide a framework for drug design". Structure. 16 (1): 82–92. doi:10.1016/j.str.2007.10.020. PMID 18184586.
- Wu N, Pai EF (Aug 2002). "Crystal structures of inhibitor complexes reveal an alternate binding mode in orotidine-5'-monophosphate decarboxylase". The Journal of Biological Chemistry. 277 (31): 28080–7. doi:10.1074/jbc.m202362200. PMID 12011084.
- Purohit MK, Poduch E, Wei LW, Crandall IE, To T, Kain KC, Pai EF, Kotra LP (Nov 2012). "Novel cytidine-based orotidine-5'-monophosphate decarboxylase inhibitors with an unusual twist". Journal of Medicinal Chemistry. 55 (22): 9988–97. doi:10.1021/jm301176r. PMID 22991951.
Further reading
- Suchi M, Harada N, Tsuboi T, Asai K, Okajima K, Wada Y, Takagi Y (1989). "Molecular Cloning of Human UMP Synthase". Purine and Pyrimidine Metabolism in Man VI. Advances in Experimental Medicine and Biology. Vol. 253A. pp. 511–8. doi:10.1007/978-1-4684-5673-8_83. ISBN 978-1-4684-5675-2. PMID 2624233.
- Suttle DP, Bugg BY, Winkler JK, Kanalas JJ (Mar 1988). "Molecular cloning and nucleotide sequence for the complete coding region of human UMP synthase". Proceedings of the National Academy of Sciences of the United States of America. 85 (6): 1754–8. Bibcode:1988PNAS...85.1754S. doi:10.1073/pnas.85.6.1754. PMC 279857. PMID 3279416.
- Patterson D, Jones C, Morse H, Rumsby P, Miller Y, Davis R (May 1983). "Structural gene coding for multifunctional protein carrying orotate phosphoribosyltransferase and OMP decarboxylase activity is located on long arm of human chromosome 3". Somatic Cell Genetics. 9 (3): 359–74. doi:10.1007/BF01539144. PMID 6574608. S2CID 29498380.
- McClard RW, Black MJ, Livingstone LR, Jones ME (Sep 1980). "Isolation and initial characterization of the single polypeptide that synthesizes uridine 5'-monophosphate from orotate in Ehrlich ascites carcinoma. Purification by tandem affinity chromatography of uridine-5'-monophosphate synthase". Biochemistry. 19 (20): 4699–706. doi:10.1021/bi00561a024. PMID 6893554.
- Iannuzzi L, Di Meo GP, Ryan AM, Gallagher DS, Ferrara L, Womack JE (May 1994). "Localization of uridine monophosphate synthase (UMPS) gene to river buffalo chromosomes by FISH". Chromosome Research. 2 (3): 255–6. doi:10.1007/BF01553326. PMID 8069469. S2CID 13407149.
- Ichikawa W, Uetake H, Shirota Y, Yamada H, Takahashi T, Nihei Z, Sugihara K, Sasaki Y, Hirayama R (Oct 2003). "Both gene expression for orotate phosphoribosyltransferase and its ratio to dihydropyrimidine dehydrogenase influence outcome following fluoropyrimidine-based chemotherapy for metastatic colorectal cancer". British Journal of Cancer. 89 (8): 1486–92. doi:10.1038/sj.bjc.6601335. PMC 2394351. PMID 14562021.
- Miyoshi Y, Uemura H, Ishiguro H, Kitamura H, Nomura N, Danenberg PV, Kubota Y (2005). "Expression of thymidylate synthase, dihydropyrimidine dehydrogenase, thymidine phosphorylase, and orotate phosphoribosyl transferase in prostate cancer". Prostate Cancer and Prostatic Diseases. 8 (3): 260–5. doi:10.1038/sj.pcan.4500817. PMID 15999119.
- Ochiai T, Sugitani M, Nishimura K, Noguchi H, Okada T, Ouchi M, Yamada M, Kitajima M, Tsuruoka Y, Takahashi Y, Futagawa S (Oct 2005). "Impact of orotate phosphoribosyl transferase activity as a predictor of lymph node metastasis in gastric cancer". Oncology Reports. 14 (4): 987–92. doi:10.3892/or.14.4.987. PMID 16142362.
- Stelzl U, Worm U, Lalowski M, Haenig C, Brembeck FH, Goehler H, Stroedicke M, Zenkner M, Schoenherr A, Koeppen S, Timm J, Mintzlaff S, Abraham C, Bock N, Kietzmann S, Goedde A, Toksöz E, Droege A, Krobitsch S, Korn B, Birchmeier W, Lehrach H, Wanker EE (Sep 2005). "A human protein-protein interaction network: a resource for annotating the proteome". Cell. 122 (6): 957–68. doi:10.1016/j.cell.2005.08.029. hdl:11858/00-001M-0000-0010-8592-0. PMID 16169070. S2CID 8235923.
- Kitajima M, Takita N, Hata M, Maeda T, Sakamoto K, Kamano T, Ochiai T (Jan 2006). "The relationship between 5-fluorouracil sensitivity and single nucleotide polymorphisms of the orotate phosphoribosyl transferase gene in colorectal cancer". Oncology Reports. 15 (1): 161–5. doi:10.3892/or.15.1.161. PMID 16328050.
- Ichikawa W, Takahashi T, Suto K, Sasaki Y, Hirayama R (Jul 2006). "Orotate phosphoribosyltransferase gene polymorphism predicts toxicity in patients treated with bolus 5-fluorouracil regimen". Clinical Cancer Research. 12 (13): 3928–34. doi:10.1158/1078-0432.CCR-05-2665. PMID 16818689.
- Taomoto J, Yoshida K, Wada Y, Tanabe K, Konishi K, Tahara H, Fukushima M (2007). "Overexpression of the orotate phosphoribosyl-transferase gene enhances the effect of 5-fluorouracil on gastric cancer cell lines". Oncology. 70 (6): 458–64. doi:10.1159/000098873. PMID 17237621. S2CID 8032104.
- Nio Y, Toga T, Maruyama R, Fukushima M (Jul 2007). "Expression of orotate phosphoribosyl transferase in human pancreatic cancer: implication for the efficacy of uracil and tegafur-based adjuvant chemotherapy". Oncology Reports. 18 (1): 59–64. doi:10.3892/or.18.1.59. PMID 17549346.
- Sanada Y, Yoshida K, Ohara M, Tsutani Y (2007). "Expression of orotate phosphoribosyltransferase (OPRT) in hepatobiliary and pancreatic carcinoma". Pathology & Oncology Research. 13 (2): 105–13. CiteSeerX 10.1.1.629.7176. doi:10.1007/BF02893485. PMID 17607371. S2CID 32129544.
- Sakamoto E, Nagase H, Kobunai T, Oie S, Oka T, Fukushima M, Oka T (Nov 2007). "Orotate phosphoribosyltransferase expression level in tumors is a potential determinant of the efficacy of 5-fluorouracil". Biochemical and Biophysical Research Communications. 363 (1): 216–22. doi:10.1016/j.bbrc.2007.08.164. PMID 17854773.