Viriditoxin
 | |
| Names | |
|---|---|
| Other names
(-)-Viriditoxin (M)-Viriditoxin | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Viriditoxin (VDT[1][2]) is a secondary metabolite produced by fungi.[3] Viriditoxin is a type of mycotoxin.[1] The biosynthesis of the compound has been investigated.[3]
Occurrence
It is produced by several Aspergillus species including A. aureoluteus,[4] A. brevipes,[5] and A. viridinutans in which it was first identified in 1971.[6] It has been isolated from Paecilomyces variotii, which was obtained from Nomura's jellyfish.[2] It is also produced by Cladosporium cladosporioides.[1]
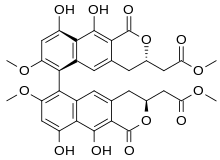
Structure
Natural viriditoxin exists as a single atropisomer owing to restricted rotation about the C-C bond which joins the two naphthol rings. It has been confirmed by total synthesis to be twisted into the so-called M isomer.[6]
Biosynthesis
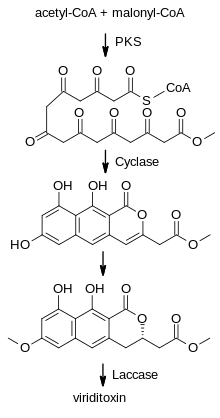
Viriditoxin is a secondary metabolite, a polyketide produced from multiple acetyl-CoA and malonyl-CoA units which are combined by a polyketide synthase (PKS) enzyme complex. A chain of eight acetate units are cyclised to give the three-ring system which forms half of the carbon framework of the final product. After selective methylation of one of the phenol groups and reduction of the pyrone ring, the resulting intermediate (semiviriditoxin) is dimerised by a laccase enzyme, generating specifically the minus M atropisomer.[7]
Uses
In nature, viriditoxin likely is used against microbial competition. On mangroves, P. variotii's production of viriditoxin was linked to antagonism against bacteria.[3]
A 2022 study found that it had potential as a leukemia treatment due to high cytotoxic potential and rapid kinetics of caspase activation when reacting to Jurkat leukemia and especially Ramos lymphoma cells; solid tumor cells were affected to a much lesser extent.[1] Tests of viriditoxin on pregnant mice found little effect on the survival of their pups and there was no increase of abnormalities.[8]
References
- Stuhldreier, Fabian; Schmitt, Laura; Lenz, Thomas; Hinxlage, Ilka; Zimmermann, Marcel; Wollnitzke, Philipp; Schliehe-Diecks, Julian; Liu, Yang; Jäger, Paul; Geyh, Stefanie; Teusch, Nicole; Peter, Christoph; Bhatia, Sanil; Haas, Rainer; Levkau, Bodo (November 8, 2022). "The mycotoxin viriditoxin induces leukemia- and lymphoma-specific apoptosis by targeting mitochondrial metabolism". Cell Death & Disease. 13 (11): 938. doi:10.1038/s41419-022-05356-w. PMC 9643474. PMID 36347842. S2CID 253387328.
 This article incorporates text from this free content work. Licensed under Creative Commons Attribution 4.0.
This article incorporates text from this free content work. Licensed under Creative Commons Attribution 4.0. - Kundu, Soma; Kim, Tae Hyung; Yoon, Jung Hyun; Shin, Han-Seung; Lee, Jaewon; Jung, Jee H.; Kim, Hyung Sik (December 1, 2014). "Viriditoxin regulates apoptosis and autophagy via mitotic catastrophe and microtubule formation in human prostate cancer cells". International Journal of Oncology. 45 (6): 2331–2340. doi:10.3892/ijo.2014.2659. PMID 25231051.
- Urquhart, Andrew S.; Hu, Jinyu; Chooi, Yit-Heng; Idnurm, Alexander (2019). "The fungal gene cluster for biosynthesis of the antibacterial agent viriditoxin". Fungal Biology and Biotechnology. 6: 2. doi:10.1186/s40694-019-0072-y. PMC 6600887. PMID 31304040.
 This article incorporates text from this free content work. Licensed under Creative Commons Attribution 4.0.
This article incorporates text from this free content work. Licensed under Creative Commons Attribution 4.0. - Samson, R.A.; Hong, S.; Peterson, S.W.; Frisvad, J.C.; Varga, J. (2007). "Polyphasic taxonomy of Aspergillus section Fumigati and its teleomorph Neosartorya". Studies in Mycology. 59: 147–203. doi:10.3114/sim.2007.59.14. PMC 2275200. PMID 18490953.
- Frederic, Lamoth; William J., Steinbach (2016). Advances in Aspergillus fumigatus pathobiology. Frontiers Media SA. ISBN 978-2-889-19789-7.
- Smyth, Jamie E.; Butler, Nicholas M.; Keller, Paul A. (2015). "A twist of nature – the significance of atropisomers in biological systems". Natural Product Reports. 32 (11): 1562–1583. doi:10.1039/c4np00121d. PMID 26282828. Archived from the original on January 28, 2023. Retrieved June 12, 2023.
- Hüttel, Wolfgang; Müller, Michael (2021). "Regio- and stereoselective intermolecular phenol coupling enzymes in secondary metabolite biosynthesis". Natural Product Reports. 38 (5): 1017–1020. doi:10.1039/d0np00010h. PMID 33196733. S2CID 226987404.
- Hood, R. D.; Hayes, A. W.; Scammell, J. G. (January 1, 1976). "Effects of prenatal administration of citrinin and viriditoxin to mice". Food and Cosmetics Toxicology. 14 (3): 175–178. doi:10.1016/S0015-6264(76)80419-1. PMID 950209.