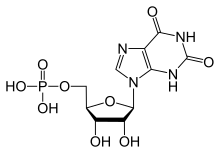
Xanthosine monophosphate
Xanthosine monophosphate also called Xanthylate is an intermediate in purine metabolism.[1] It is a ribonucleoside monophosphate. It is formed from IMP via the action of IMP dehydrogenase, and it forms GMP via the action of GMP synthase. Also, XMP can be released from XTP by enzyme deoxyribonucleoside triphosphate pyrophosphohydrolase containing (d)XTPase activity.[2]
 | |
| Names | |
|---|---|
| IUPAC name
5'-xanthylic acid | |
| Other names
xanthine ribotide, XMP | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
| MeSH | Xanthosine+monophosphate |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H13N4O9P | |
| Molar mass | 364.206 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
It is abbreviated XMP.[3]
See also
References
- McMurry, John (2007). Organic chemistry: a biological approach. Cengage Learning. pp. 1007–. ISBN 9780495015253. Retrieved 26 March 2012.
- Davies O, Mendes P, Smallbone K, Malys N (2012). "Characterisation of multiple substrate-specific (d)ITP/(d)XTPase and modelling of deaminated purine nucleotide metabolism" (PDF). BMB Reports. 45 (4): 259–64. doi:10.5483/BMBRep.2012.45.4.259. PMID 22531138.
- Gogia, S.; Balaram, H.; Puranik, M. (May 2011). "Hypoxanthine guanine phosphoribosyltransferase distorts the purine ring of nucleotide substrates and perturbs the pKa of bound xanthosine monophosphate". Biochemistry. 50 (19): 4184–93. doi:10.1021/bi102039b. PMID 21486037.
Further reading
- Sigel, H; Operschall, BP; Griesser, R (2009). "Xanthosine 5'-monophosphate (XMP). Acid-base and metal ion-binding properties of a chameleon-like nucleotide" (PDF). Chemical Society Reviews. 38 (8): 2465–94. doi:10.1039/b902181g. PMID 19623361. S2CID 205726340. Archived from the original (PDF) on 2020-01-01.
- Egli, M; Pallan, PS (2010). "Crystallographic studies of chemically modified nucleic acids: A backward glance". Chemistry & Biodiversity. 7 (1): 60–89. doi:10.1002/cbdv.200900177. PMC 2905155. PMID 20087997.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.