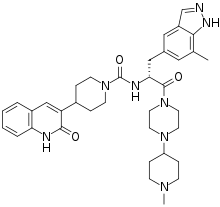
Zavegepant
Zavegepant, sold under the brand name Zavzpret, is a medication used for the treatment of migraine.[1] Zavegepant is a calcitonin gene-related peptide receptor antagonist.[1] It is sprayed into the nose.[1] It is sold by Pfizer.[1]
 | |
| Clinical data | |
|---|---|
| Trade names | Zavzpret |
| Other names | BHV-3500 |
| License data |
|
| Routes of administration | Nasal |
| Drug class | Calcitonin gene-related peptide receptor antagonist |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEMBL |
|
| Chemical and physical data | |
| Formula | C36H46N8O3 |
| Molar mass | 638.817 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
The most common adverse reactions include taste disorders, nausea, nasal discomfort, and vomiting.[1]
Zavegepant was approved for medical use in the United States in March 2023.[1][2][3]
Medical uses
Zavegepant is indicated for the acute treatment of migraine with or without aura in adults.[1]
References
- "Zavzpret- zavegepant spray". DailyMed. 9 March 2023. Retrieved 25 August 2023.
- "Drug Approval Package: Zavzpret". U.S. Food and Drug Administration (FDA). 3 April 2023. Retrieved 25 August 2023.
- "Pfizer's Zavzpret (Zavegepant) Migraine Nasal Spray Receives FDA Approval" (Press release). 10 March 2023.
Further reading
- Croop R, Madonia J, Stock DA, Thiry A, Forshaw M, Murphy A, Coric V, Lipton RB (October 2022). "Zavegepant nasal spray for the acute treatment of migraine: A Phase 2/3 double-blind, randomized, placebo-controlled, dose-ranging trial". Headache. 62 (9): 1153–1163. doi:10.1111/head.14389. PMC 9827820. PMID 36239038.
- Noor N, Angelette A, Lawson A, Patel A, Urits I, Viswanath O, et al. (2022). "A Comprehensive Review of Zavegepant as Abortive Treatment for Migraine". Health Psychology Research. 10 (3): 35506. doi:10.52965/001c.35506. PMC 9239361. PMID 35774914.
- Scuteri D, Tarsitano A, Tonin P, Bagetta G, Corasaniti MT (November 2022). "Focus on zavegepant: the first intranasal third-generation gepant". Pain Management. 12 (8): 879–885. doi:10.2217/pmt-2022-0054. PMID 36189708. S2CID 252681912.
External links
- Clinical trial number NCT04571060 for "Randomized Trial in Adult Subjects With Acute Migraines" at ClinicalTrials.gov
- Clinical trial number NCT03872453 for "Acute Treatment Trial in Adult Subjects With Migraines" at ClinicalTrials.gov
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.