Androgen backdoor pathway
The androgen backdoor pathway is a collective name for all metabolic pathways where clinically relevant androgens are synthesized with roundabout of testosterone as an intermediate product. Initially described as pathway where 5α-reduction of 17α-hydroxyprogesterone ultimately leads to 5α-dihydrotestosterone,[1] several other pathways have been since then discovered that lead to 11-oxyandrogens which are potent agonists of the androgen receptors.[2] A backdoor pathway is an alternative to the conventional,[3] canonical[4] androgenic pathway that involves testosterone.
Dihydrotestosterone
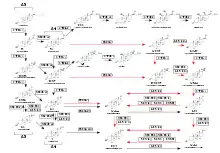
The primary feature of the androgen backdoor pathway is that 17α-hydroxyprogesterone (17-OHP) can be 5α-reduced and finally converted to 5α-dihydrotestosterone (DHT) via an alternative route that bypasses the conventional[3] intermediates androstenedione and testosterone.[1][6]
In mammals, this route is activated during normal prenatal development and leads to early male sexual differentiation.[7][8][9] It was first described in the marsupials and later confirmed in humans.[10]
In 21-hydroxylase deficiency[6] or cytochrome P450 oxidoreductase deficiency,[11] this route may be activated regardless of age and sex by even a mild increase in circulating 17-OHP levels.[12][13]
The first step of this route is 5α-reduction of 17-OHP by SRD5A1/SRD5A2 enzymes to 5α-pregnan-17α-ol-3,20-dione.[14][11][6][7] The next two intermediate products are 5α-pregnane-3α,17α-diol-20-one and androsterone.[4][12][15][14] The final step is conversion of 5α-androstane-3α,17β-diol (androstanediol) to DHT by several 3α-oxidoreductases (HSD17B6, RDH16, etc.).[11][15] Hence, androstanediol is a marker of the backdoor pathway of DHT synthesis.[16]
Therefore, the pathway can be outlined as 17-OHP → 5α-pregnan-17α-ol-3,20-dione → 5α-pregnane-3α,17α-diol-20-one → androsterone → androstanediol → DHT.[17]
11-Oxyandrogens
Another feature of the backdoor pathway is production of 11-oxygenated (oxygen atom on C11 position forms a ketone group) 19-carbon steroids, also termed 11-oxyandrogens: 11-ketotestosterone and 11-ketodihydrotestosterone, which are 11-keto forms of testosterone and DHT, respectively. The synthesis of 11-oxyandrogens in this pathway does not require testosterone or DHT as intermediate products. 11-oxyandrogens are potent and clinically relevant agonists of the androgen receptors.[2] Potency of 11-ketotestosterone is similar to that of testosterone.[18] 11-ketotestosterone may serve as the main androgen for healthy women.[19]
11-oxyandrogens may be produced in physiologic quantities in healthy organisms,[19] and in excessive quantities in the pathological conditions like 21-hydroxylase deficiency,[20][13][21] polycystic ovary syndrome,[22] benign prostatic hyperplasia[23] in prostate cancer[24] and disorders of sex development in neonates and in children.[25]
In 21-hydroxylase deficiency, the steroid 11β-hydroxylase (11βOH) enzyme, also known is CYP11B1, stays at the initial step of 11-oxyandrogen production.
There are several routes that may lead to production of 11-oxyandrogens:
- 17-OHP is converted by 11βOH to 21-deoxycortisol, which is subsequently converted to 11-ketotestosterone and 11-ketodihydrotestosterone.[26][27]
- Progesterone is converted by 11βOH to 11β-hydroxyprogesterone.[28] There are multiple studies conducted since 1987 that demonstrate increased levels of 11β-hydroxyprogesterone in congenital adrenal hyperplasia.[29][30][31] In vitro studies predict that excess of 11β-hydroxyprogesterone may ultimately leads to production of 11-ketodihydrotestosterone and 11-ketoandrosterone.[32][28][23]
- Androstenedione is converted by 11βOH to 11β-hydroxyandrostenedione and then to 11-ketotestosterone and 11-ketodihydrotestosterone.[23][13]
Clinical significance
Unlike testosterone and androstenedione, androgens produced by the backdoor pathway, i.e. DHT and 11-oxyandrogens, cannot be converted by aromatase into estrogens.[33]
The backdoor pathway is not always considered in the clinical evaluation of patients with hyperandrogenism. Ignoring this pathway may lead to diagnostic pitfalls and confusion, for example, in late onset congenital adrenal hyperplasia, where testosterone levels may be normal amid the symptoms of hyperandrogenism like hirsutism and acne.[12]
History
In April 1987, Benjamin Eckstein and colleagues reported that androstanediol, a direct precursor to DHT, is synthesized in immature rat testes in a pathway that predominantly involves 17-OHP but not androstenedione as an intermediate.[34]
In October 2000, Geoffrey Shaw and colleagues demonstrated that prostate formation in a marsupial (tammar wallaby pouch young) was mediated by the testicular androgen androstanediol, which is higher in male than in female plasma during early sexual differentiation, identifying it as a key hormone in male development. They have shown that androstanediol acts in target tissues via DHT, i.e. is converted to DHT in target tissues, so that testosterone is not the only source of DHT.[8]
In February 2003, Jean Wilson and colleagues described that DHT, a 5α-reduced androgen, can be synthesized from 17-OHP by two pathways: with and without testosterone as an intermediate. They have demonstrated that androstanediol, a precursor to DHT, is formed in the testes of tammar wallaby pouch young with 5α-pregnane-3α,17α-diol-20-one and androsterone as intermediates.[35]
In July 2004, Mala Mahendroo and colleagues described that androstanediol is the predominant androgen in immature mouse testes, and that it is formed by two pathways; the main one involves testosterone, and a second utilizes the pathway progesterone → 5α-dihydroprogesterone → 5α-pregnane-3α-ol-20-one (allopregnanolone) → 5α-pregnane-3α,17α-diol-20-one → androsterone → androstanediol.[9]
In November 2004, Richard Auchus coined the term "backdoor pathway" in a review called "The backdoor pathway to dihydrotestosterone". He defined the backdoor pathway as a "route to DHT that does not involve the testosterone intermediate". He emphasized that this alternative pathway seems to explain how potent androgens are produced under certain normal and pathological conditions when the conventional androgen biosynthetic pathway cannot fully explain the observed consequences.[1]
See also
References
- Auchus RJ (November 2004). "The backdoor pathway to dihydrotestosterone". Trends in Endocrinology and Metabolism. 15 (9): 432–8. doi:10.1016/j.tem.2004.09.004. PMID 15519890. S2CID 10631647.
- Turcu AF, Nanba AT, Auchus RJ (2018). "The Rise, Fall, and Resurrection of 11-Oxygenated Androgens in Human Physiology and Disease". Hormone Research in Paediatrics. 89 (5): 284–291. doi:10.1159/000486036. PMC 6031471. PMID 29742491.
- Kinter, K. J.; Anekar, A. A. (2021). Biochemistry, Dihydrotestosterone. StatPearls. PMID 32491566.
- O'Shaughnessy PJ, Antignac JP, Le Bizec B, Morvan ML, Svechnikov K, Söder O, Savchuk I, Monteiro A, Soffientini U, Johnston ZC, Bellingham M, Hough D, Walker N, Filis P, Fowler PA (February 2019). "Alternative (backdoor) androgen production and masculinization in the human fetus". PLOS Biology. 17 (2): e3000002. doi:10.1371/journal.pbio.3000002. PMC 6375548. PMID 30763313.
androsterone as the predominant backdoor androgen in the human fetus
- Miller, WL; Auchus, RJ (April 2019). "The "backdoor pathway" of androgen synthesis in human male sexual development". PLOS Biology. 17 (4): e3000198. doi:10.1371/journal.pbio.3000198. PMC 6464227. PMID 30943210.
- Kamrath C, Hochberg Z, Hartmann MF, Remer T, Wudy SA (March 2012). "Increased activation of the alternative "backdoor" pathway in patients with 21-hydroxylase deficiency: evidence from urinary steroid hormone analysis". The Journal of Clinical Endocrinology and Metabolism. 97 (3): E367–75. doi:10.1210/jc.2011-1997. PMID 22170725.
- Miller WL, Auchus RJ (April 2019). "The "backdoor pathway" of androgen synthesis in human male sexual development". PLOS Biology. 17 (4): e3000198. doi:10.1371/journal.pbio.3000198. PMC 6464227. PMID 30943210.
- Shaw G, Renfree MB, Leihy MW, Shackleton CH, Roitman E, Wilson JD (October 2000). "Prostate formation in a marsupial is mediated by the testicular androgen 5 alpha-androstane-3 alpha,17 beta-diol". Proceedings of the National Academy of Sciences of the United States of America. 97 (22): 12256–9. Bibcode:2000PNAS...9712256S. doi:10.1073/pnas.220412297. PMC 17328. PMID 11035809.
- Mahendroo M, Wilson JD, Richardson JA, Auchus RJ (July 2004). "Steroid 5alpha-reductase 1 promotes 5alpha-androstane-3alpha,17beta-diol synthesis in immature mouse testes by two pathways". Molecular and Cellular Endocrinology. 222 (1–2): 113–20. doi:10.1016/j.mce.2004.04.009. PMID 15249131. S2CID 54297812.
- Flück CE, Meyer-Böni M, Pandey AV, Kempná P, Miller WL, Schoenle EJ, Biason-Lauber A (August 2011). "Why boys will be boys: two pathways of fetal testicular androgen biosynthesis are needed for male sexual differentiation". American Journal of Human Genetics. 89 (2): 201–18. doi:10.1016/j.ajhg.2011.06.009. PMC 3155178. PMID 21802064.
- Reisch N, Taylor AE, Nogueira EF, Asby DJ, Dhir V, Berry A, Krone N, Auchus RJ, Shackleton CH, Hanley NA, Arlt W (October 2019). "Alternative pathway androgen biosynthesis and human fetal female virilization". Proceedings of the National Academy of Sciences of the United States of America. 116 (44): 22294–22299. doi:10.1073/pnas.1906623116. PMC 6825302. PMID 31611378.
- Sumińska, Marta; Bogusz-Górna, Klaudia; Wegner, Dominika; Fichna, Marta (29 June 2020). "Non-Classic Disorder of Adrenal Steroidogenesis and Clinical Dilemmas in 21-Hydroxylase Deficiency Combined with Backdoor Androgen Pathway. Mini-Review and Case Report". International Journal of Molecular Sciences. 21 (13): 4622. doi:10.3390/ijms21134622. PMC 7369945. PMID 32610579.
- Pignatelli, Duarte; Pereira, Sofia S.; Pasquali, Renato (2019). "Androgens in Congenital Adrenal Hyperplasia". Hyperandrogenism in Women. Frontiers of Hormone Research. Vol. 53. pp. 65–76. doi:10.1159/000494903. ISBN 978-3-318-06470-4. PMID 31499506. S2CID 202412336.
- Fukami M, Homma K, Hasegawa T, Ogata T (April 2013). "Backdoor pathway for dihydrotestosterone biosynthesis: implications for normal and abnormal human sex development". Developmental Dynamics. 242 (4): 320–9. doi:10.1002/dvdy.23892. PMID 23073980. S2CID 44702659.
- Auchus, Richard J. (2010). "Management of the Adult with Congenital Adrenal Hyperplasia". International Journal of Pediatric Endocrinology. 2010: 614107. doi:10.1155/2010/614107. PMC 2896848. PMID 20613954.
- Rohayem J, Zitzmann M, Laurentino S, Kliesch S, Nieschlag E, Holterhus PM, Kulle A (September 2020). "The role of gonadotropins in testicular and adrenal androgen biosynthesis pathways-Insights from males with congenital hypogonadotropic hypogonadism on hCG/rFSH and on testosterone replacement". Clinical Endocrinology. 94 (1): 90–101. doi:10.1111/cen.14324. PMID 32871622.
- Sharifi N, McPhaul MJ, Auchus RJ (December 2010). ""Getting from here to there"--mechanisms and limitations to the activation of the androgen receptor in castration-resistant prostate cancer". Journal of Investigative Medicine. 58 (8): 938–44. doi:10.2310/JIM.0b013e3181ff6bb8. PMC 5589138. PMID 21030877.
- Turcu AF, Rege J, Auchus RJ, Rainey WE (May 2020). "11-Oxygenated androgens in health and disease". Nature Reviews. Endocrinology. 16 (5): 284–296. doi:10.1038/s41574-020-0336-x. PMC 7881526. PMID 32203405. S2CID 212732699.
- Barnard L, Nikolaou N, Louw C, Schiffer L, Gibson H, Gilligan LC, Gangitano E, Snoep J, Arlt W, Tomlinson JW, Storbeck KH (September 2020). "The A-ring reduction of 11-ketotestosterone is efficiently catalysed by AKR1D1 and SRD5A2 but not SRD5A1". The Journal of Steroid Biochemistry and Molecular Biology. 202: 105724. doi:10.1016/j.jsbmb.2020.105724. PMID 32629108. S2CID 220323715.
- Turcu AF, Mallappa A, Elman MS, Avila NA, Marko J, Rao H, Tsodikov A, Auchus RJ, Merke DP (August 2017). "11-Oxygenated Androgens Are Biomarkers of Adrenal Volume and Testicular Adrenal Rest Tumors in 21-Hydroxylase Deficiency". The Journal of Clinical Endocrinology and Metabolism. 102 (8): 2701–2710. doi:10.1210/jc.2016-3989. PMC 5546849. PMID 28472487.
- White PC (June 2018). "Update on diagnosis and management of congenital adrenal hyperplasia due to 21-hydroxylase deficiency". Current Opinion in Endocrinology, Diabetes and Obesity. 25 (3): 178–184. doi:10.1097/MED.0000000000000402. PMID 29718004. S2CID 26072848.
- Gent R, du Toit T, Bloem LM, Swart AC (May 2019). "The 11β-hydroxysteroid dehydrogenase isoforms: pivotal catalytic activities yield potent C11-oxy C19 steroids with 11βHSD2 favouring 11-ketotestosterone, 11-ketoandrostenedione and 11-ketoprogesterone biosynthesis". The Journal of Steroid Biochemistry and Molecular Biology. 189: 116–126. doi:10.1016/j.jsbmb.2019.02.013. PMID 30825506. S2CID 73490363.
- du Toit T, Swart AC (February 2020). "The 11β-hydroxyandrostenedione pathway and C11-oxy C21 backdoor pathway are active in benign prostatic hyperplasia yielding 11keto-testosterone and 11keto-progesterone". The Journal of Steroid Biochemistry and Molecular Biology. 196: 105497. doi:10.1016/j.jsbmb.2019.105497. PMID 31626910. S2CID 204734045.
- Storbeck KH, Mostaghel EA (2019). "Canonical and Noncanonical Androgen Metabolism and Activity". Advances in Experimental Medicine and Biology. 1210: 239–277. doi:10.1007/978-3-030-32656-2_11. ISBN 978-3-030-32655-5. PMID 31900912. S2CID 209748543.
- du Toit T, Stander MA, Swart AC (March 2018). "A high-throughput UPC2-MS/MS method for the separation and quantification of C19 and C21 steroids and their C11-oxy steroid metabolites in the classical, alternative, backdoor and 11OHA4 steroid pathways". Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences. 1080: 71–81. doi:10.1016/j.jchromb.2018.02.023. PMID 29482121.
- Nordenström A, Falhammar H (March 2019). "MANAGEMENT OF ENDOCRINE DISEASE: Diagnosis and management of the patient with non-classic CAH due to 21-hydroxylase deficiency". European Journal of Endocrinology. 180 (3): R127–R145. doi:10.1530/EJE-18-0712. PMID 30566904.
- Barnard L, Gent R, van Rooyen D, Swart AC (November 2017). "Adrenal C11-oxy C21 steroids contribute to the C11-oxy C19 steroid pool via the backdoor pathway in the biosynthesis and metabolism of 21-deoxycortisol and 21-deoxycortisone". The Journal of Steroid Biochemistry and Molecular Biology. 174: 86–95. doi:10.1016/j.jsbmb.2017.07.034. PMID 28774496. S2CID 24071400.
- van Rooyen, Desmaré; Gent, Rachelle; Barnard, Lise; Swart, Amanda C. (April 2018). "The in vitro metabolism of 11β-hydroxyprogesterone and 11-ketoprogesterone to 11-ketodihydrotestosterone in the backdoor pathway". The Journal of Steroid Biochemistry and Molecular Biology. 178: 203–212. doi:10.1016/j.jsbmb.2017.12.014. PMID 29277707. S2CID 3700135.
- Gueux, Bernard; Fiet, Jean; Galons, Hervé; Boneté, Rémi; Villette, Jean-Marie; Vexiau, Patrick; Pham-Huu-Trung, Marie-Thérèse; Raux-Eurin, Marie-Charles; Gourmelen, Micheline; Brérault, Jean-Louis; Julien, René; Dreux, Claude (January 1987). "The measurement of 11β-hydroxy-4-pregnene-3,20-dione (21-Deoxycorticosterone) by radioimmunoassay in human plasma". Journal of Steroid Biochemistry. 26 (1): 145–150. doi:10.1016/0022-4731(87)90043-4. PMID 3546944.
- Fiet, Jean; Gueux, Bernard; Rauxdemay, Marie-Charles; Kuttenn, Frederique; Vexiau, Patrick; Brerault, Jeanlouis; Couillin, Philippe; Galons, Herve; Villette, Jeanmarie; Julien, Rene; Dreux, Claude (March 1989). "Increased Plasma 21-Deoxycorticosterone (21-DB) Levels in Late-Onset Adrenal 21-Hydroxylase Deficiency Suggest a Mild Defect of the Mineralocorticoid Pathway". The Journal of Clinical Endocrinology & Metabolism. 68 (3): 542–547. doi:10.1210/jcem-68-3-542. PMID 2537337.
- Fiet, Jean; Le Bouc, Yves; Guéchot, Jérôme; Hélin, Nicolas; Maubert, Marie-Anne; Farabos, Dominique; Lamazière, Antonin (10 February 2017). "A Liquid Chromatography/Tandem Mass Spectometry [sic] Profile of 16 Serum Steroids, Including 21-Deoxycortisol and 21-Deoxycorticosterone, for Management of Congenital Adrenal Hyperplasia". Journal of the Endocrine Society. 1 (3): 186–201. doi:10.1210/js.2016-1048. PMC 5686660. PMID 29264476.
- van Rooyen D, Yadav R, Scott EE, Swart AC (May 2020). "CYP17A1 exhibits 17αhydroxylase/17,20-lyase activity towards 11β-hydroxyprogesterone and 11-ketoprogesterone metabolites in the C11-oxy backdoor pathway". The Journal of Steroid Biochemistry and Molecular Biology. 199: 105614. doi:10.1016/j.jsbmb.2020.105614. PMID 32007561. S2CID 210955834.
- Nagasaki, Keisuke; Takase, Kaoru; Numakura, Chikahiko; Homma, Keiko; Hasegawa, Tomonobu; Fukami, Maki (30 August 2020). "Foetal virilisation caused by overproduction of non-aromatisable 11-oxygenated C19 steroids in maternal adrenal tumour". Human Reproduction. 35 (11): 2609–2612. doi:10.1093/humrep/deaa221. PMID 32862221.
- Eckstein B, Borut A, Cohen S (April 1987). "Metabolic pathways for androstanediol formation in immature rat testis microsomes". Biochimica et Biophysica Acta (BBA) - General Subjects. 924 (1): 1–6. doi:10.1016/0304-4165(87)90063-8. PMID 3828389.
- Wilson JD, Auchus RJ, Leihy MW, Guryev OL, Estabrook RW, Osborn SM, Shaw G, Renfree MB (February 2003). "5alpha-androstane-3alpha,17beta-diol is formed in tammar wallaby pouch young testes by a pathway involving 5alpha-pregnane-3alpha,17alpha-diol-20-one as a key intermediate". Endocrinology. 144 (2): 575–80. doi:10.1210/en.2002-220721. PMID 12538619.