Sexual differentiation
Sexual differentiation is the process of development of the sex differences between males and females from an undifferentiated zygote.[1][2] Sex determination is often distinct from sex differentiation; sex determination is the designation for the development stage towards either male or female, while sex differentiation is the pathway towards the development of the phenotype.[3]
| Sexual differentiation | |
|---|---|
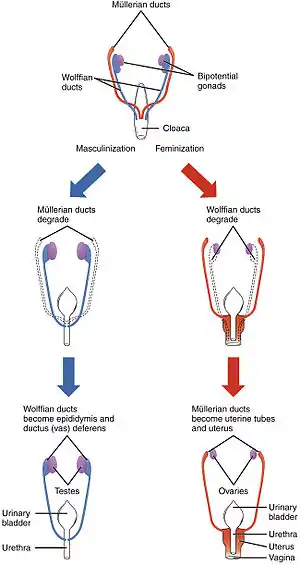 Differentiation of the male and female reproductive systems does not occur until the fetal period of development. | |
| Anatomical terminology |
In many species, testicular or ovarian differentiation begins with appearance of Sertoli cells in males and granulosa cells in females.[4]
As male and female individuals develop from embryos into mature adults, sex differences at many levels develop, such as genes, chromosomes, gonads, hormones, anatomy, and psyche. Beginning with determination of sex by genetic and/or environmental factors, humans and other organisms proceed down different pathways of differentiation as they grow and develop. These processes are not fixed, and can change over one organism's lifetime or over many generations evolutionarily.[4]
Sex determination systems
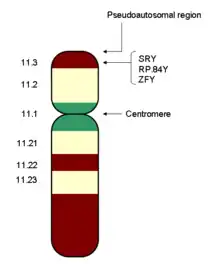
Humans, many mammals, insects and other animals have an XY sex-determination system. Humans have forty-six chromosomes, including two sex chromosomes, XX in females and XY in males. The Y chromosome must carry at least one essential gene which determines testicular formation (originally termed TDF). A gene in the sex-determining region of the short arm of the Y, now referred to as SRY, has been found to direct production of a protein, testis determining factor, which binds to DNA, inducing differentiation of cells derived from the genital ridges into testes.[5] In transgenic XX mice (and some human XX males), SRY alone is sufficient to induce male differentiation.[6]
Other chromosomal systems exist in other taxa, such as the ZW sex-determination system in birds[7] and the XO system in insects.[8]
Environmental sex determination refers to the determination (and then differentiation) of sex via non-genetic cues like social factors, temperature, and available nutrients. In some species, such as the hermaphroditic clownfish, sex differentiation can occur more than once as a response to different environmental cues,[9] offering an example of how sex differentiation does not always follow a typical linear path.
There have been multiple transitions between environmental and genetic sex determination systems in reptiles over time,[10] and recent studies have shown that temperature can sometimes override sex determination via chromosomes.[11]
Humans
The early stages of human differentiation appear to be quite similar to the same biological processes in other mammals and the interaction of genes, hormones and body structures is fairly well understood. In the first weeks of gestation, a fetus has no anatomic or hormonal sex, and only a karyotype distinguishes male from female. Specific genes induce gonadal differences, which produce hormonal differences, which cause anatomic differences, leading to psychological and behavioral differences, some of which are innate and some induced by the social environment.
Various processes are involved in the development of sex differences in humans. Sexual differentiation in humans includes development of different genitalia and the internal genital tracts, breasts, body hair, and plays a role in gender identification.[12]
The development of sexual differences begins with the XY sex-determination system that is present in humans, and complex mechanisms are responsible for the development of the phenotypic differences between male and female humans from an undifferentiated zygote.[13] Atypical sexual development, and ambiguous genitalia, can be a result of genetic and hormonal factors.[14]
The differentiation of other parts of the body than the sex organ creates the secondary sex characteristics. Sexual dimorphism of skeletal structure develops during childhood, and becomes more pronounced at adolescence. Sexual orientation has been demonstrated to correlate with skeletal characters that become dimorphic during early childhood (such as arm length to stature ratio) but not with characters that become dimorphic during puberty—such as shoulder width.[15]
Other animals
The first genes involved in the cascade of differentiation can differ between taxa and even between closely related species. For example: in zebrafish the first known gene to induce male differentiation is the amh gene, in tilapia it is tDmrt1, and in southern catfish it is foxl2.[16]
In fish, due to the fact that modes of reproduction range from gonochorism (distinct sexes) to self-fertilizing hermaphroditism (where one organism has functioning gonadal features of multiple sexes), sexual differentiation is complex. Two major pathways in gonochores exist: one with a nonfunctional intersexual phase leading to delayed differentiation (secondary), and one without (primary), where differences between the sexes can be noted prior to hatching.[4] Secondary gonochorists remain in the intersex phase until a biotic or abiotic cue directs development down one pathway. Primary gonochorism, without an intersex phase, follows classical pathways of genetic sex determination, but can still be later influenced by the environment.[4] Differentiation pathways progress, and secondary sex characteristics such as anal fin bifurcation and ornamentation typically arise at puberty.[16]
In birds, thanks to research on Gallus gallus domesticus, it has been shown that determination of sex is likely cell-autonomous, i.e. that sex is determined in each somatic cell independently of, or in conjunction with, the hormone signaling that occurs in other species.[17] Studies on gynandromorph chickens showed that the mosaicism could not be explained by hormones alone, pointing to direct genetic factors, possibly one or a few Z-specific genes such as double-sex or DMRT1.[17]
In cattle, freemartins have intersex development.
Flexibility
The most intensively studied species, such as fruit flies, nematodes, and mice, reveal that evolutionarily, sex determination/differentiation systems are not wholly conserved and have evolved over time.[10] Beyond the presence or absence of chromosomes or social/environmental factors, sexual differentiation can be regulated in part by complex systems like the ratio of genes on X chromosomes and autosomes, protein production and transcription, and specific mRNA splicing.[10]
Differentiation pathways can be altered at many stages of the process. Sex reversal, where the development of a sexual phenotype is redirected during embryonic development, happens in the initiation phase of gonadal sex differentiation. Even in species where there is a well-documented master regulator gene, its effects can be overridden by a downstream gene.[18]
Furthermore, hermaphrodites serve as examples of the flexibility of sexual differentiation systems. Sequential hermaphrodites are organisms that possess reproductive capabilities of one sex, and then that sex changes.[19] Differentiated gonadal tissue of the organism's former sex degenerates, and new sex gonadal tissue grows and differentiates.[9] Organisms that have the physiological capability to reproduce as a male and as a female at the same time are known as simultaneous hermaphrodites. Some simultaneous hermaphroditic organisms, like certain species of goby, have distinctive male and female phases of reproduction and can flip back and forth, or "sex reverse", between the two.[20]
Socially-determined
In some species, such as sequentially hermaphroditic clownfish, changes in social environment can lead to sexual differentiation or sex reversal, i.e. differentiation in the opposite direction.[9] In clownfish, females are larger than males, and in social groups, there is typically one large female, multiple smaller males, and undifferentiated juveniles. If the female is removed from the group, the largest male changes sex, i.e. the former gonad tissue degenerates and new gonad tissue grows. Furthermore, the pathway of differentiation in activated in the largest juvenile, which becomes male.[9]
Alternative morphs
Sexual differentiation in a species does not have to produce one recognizable female type and one recognizable male type. In some species alternative morphs, or morphotypes, within one sex exist, such as flanged (larger than females, with large flap-like cheek-pads) and unflanged (about the same size as females, no cheek-pads) male orangutans,[21] and sometimes differences between male morphs can be more noticeable than differences between a male and a female within such species.[22] Furthermore, sexual selection can be involved in the development of different types of males with alternative reproductive strategies, such as sneaker and territorial males in dung beetles[23] or haremic males and pair-bonding males in the Nigerian cichlid fish P. pulcher.[16][24] Sometimes alternative morphs are produced by genetic differences, and in other cases, the environment can be involved, demonstrating some degree of phenotypic plasticity.[25]
Brain differentiation
In many animals, differences in the exposure of a fetal brain to sex hormones are correlated with significant differences of brain structure and function, which correlate with adult reproductive behavior.[5] The causes of differences between the sexes are only understood in some species. Fetal sex differences in human brains coupled with early differences in experience may be responsible for sex differences observed in children between 4 years old and adolescence.[26]
Many individual studies in humans and other primates have found statistically significant sex differences in specific brain structures; however, some studies have found no sex differences, and some meta-analyses have called into question the over-generalization that women and men's brains function differently.[27] Males and females statistically differ in some aspects of their brains, but there are areas of the brain which appear not to be sexually differentiated at all. Some scholars describe human brain variation not as two distinct categories, but as occupying a place on a maleness-femaleness continuum.[28]
In birds, hypotheses of male-female brain sex differences have been challenged by recent findings that differences between groups can be at least partially explained by the individual's dominance rank.[29] Furthermore, the behavioral causes of brain sex differences have been enumerated in studies of sex differences between different mating systems. For example, males of a polygynous vole species with intrasexual male competition have better spatial learning and memory than the females of their own species, but also better spatial learning and memory than all sexes of other closely related species that are monogamous; thus the brain differences commonly seen as "sex differences" have been instead linked to competition.[30] Sexual selection does play a role in some species, though, as males who display more song behaviors are selected for by females—so some sex differences in bird song brain regions seem to have been evolutionarily selected for over time.[30]
References
- Beukeboom, Leo W.; Perrin, Nicolas (2014). The Evolution of Sex Determination. Oxford University Press. p. 158. ISBN 978-0199657148.
- Koob, George F. (2010). Encyclopedia of Behavioral Neuroscience. Elsevier. p. 21. ISBN 978-0080914558.
- Beukeboom LW, Perrin N (2014). The Evolution of Sex Determination. Oxford University Press. p. 16. ISBN 978-0-19-965714-8.
- Pandian, T. J. (2013-05-07). Endocrine Sex Differentiation in Fish. CRC Press. doi:10.1201/b14771. ISBN 978-0-429-10222-6.
- Wilhelm, Dagmar; Palmer, Stephen; Koopman, Peter (2007-01-01). "Sex Determination and Gonadal Development in Mammals". Physiological Reviews. 87 (1): 1–28. doi:10.1152/physrev.00009.2006. ISSN 0031-9333. PMID 17237341.
- Gilbert, Scott F. (2000). "Chromosomal Sex Determination in Mammals". Developmental Biology. 6th Edition.
- Chue, J; Smith, C (2011-01-31). "Sex Determination and Sexual Differentiation in the Avian Model". The FEBS Journal. 278 (7): 1027–34. doi:10.1111/j.1742-4658.2011.08032.x. PMID 21281451. S2CID 24751510.
- Blackmon, Heath; Ross, Laura; Bachtrog, Doris (January 2017). "Sex Determination, Sex Chromosomes, and Karyotype Evolution in Insects". Journal of Heredity. 108 (1): 78–93. doi:10.1093/jhered/esw047. ISSN 0022-1503. PMC 6281344. PMID 27543823.
- Casas, Laura; Saborido-Rey, Fran; Ryu, Taewoo; Michell, Craig; Ravasi, Timothy; Irigoien, Xabier (2016-10-17). "Sex Change in Clownfish: Molecular Insights from Transcriptome Analysis". Scientific Reports. 6: 35461. Bibcode:2016NatSR...635461C. doi:10.1038/srep35461. ISSN 2045-2322. PMC 5066260. PMID 27748421.
- Rhen, T.; Schroeder, A. (March 2010). "Molecular Mechanisms of Sex Determination in Reptiles". Sexual Development. 4 (1–2): 16–28. doi:10.1159/000282495. ISSN 1661-5425. PMC 2918650. PMID 20145384.
- Pokorná, Martina; Kratochvíl, Lukáš (2009-05-01). "Phylogeny of sex-determining mechanisms in squamate reptiles: are sex chromosomes an evolutionary trap?". Zoological Journal of the Linnean Society. 156 (1): 168–183. doi:10.1111/j.1096-3642.2008.00481.x. ISSN 0024-4082.
- "Human sexual differentiation".
- Mukherjee, Asit B.; Parsa, Nasser Z. (1990). "Determination of sex chromosomal constitution and chromosomal origin of drumsticks, drumstick-like structures, and other nuclear bodies in human blood cells at interphase by fluorescence in situ hybridization". Chromosoma. 99 (6): 432–5. doi:10.1007/BF01726695. PMID 2176962. S2CID 25732504.
- Kučinskas, Laimutis; Just, Walter (2005). "Human male sex determination and sexual differentiation: Pathways, molecular interactions and genetic disorders". Medicina. 41 (8): 633–40. PMID 16160410.
- Martin, James T; Nguyen, Duc Huu (2004). "Anthropometric analysis of homosexuals and heterosexuals: Implications for early hormone exposure". Hormones and Behavior. 45 (1): 31–9. doi:10.1016/j.yhbeh.2003.07.003. PMID 14733889. S2CID 46091140.
- Pandian, T. J. (2012-06-05). Genetic Sex Differentiation in Fish. CRC Press. doi:10.1201/b12296. ISBN 978-0-429-08641-0.
- Chue, J; Smith, C (2011-01-31). "Sex Determination and Sexual Differentiation in the Avian Model". The FEBS Journal. 278 (7): 1027–34. doi:10.1111/j.1742-4658.2011.08032.x. PMID 21281451. S2CID 24751510.
- Capel, Blanche (2017-08-14). "Vertebrate sex determination: evolutionary plasticity of a fundamental switch". Nature Reviews Genetics. 18 (11): 675–689. doi:10.1038/nrg.2017.60. ISSN 1471-0056. PMID 28804140. S2CID 4313871.
- Warner, Robert R. (1975). "The Adaptive Significance of Sequential Hermaphroditism in Animals". The American Naturalist. 109 (965): 61–82. doi:10.1086/282974. ISSN 0003-0147. JSTOR 2459637. S2CID 84279130.
- St. Mary, Colette M. (1996-02-01). "Sex allocation in a simultaneous hermaphrodite, the zebra goby Lythrypnus zebra: insights gained through a comparison with its sympatric congener, Lythrypnus dalli". Environmental Biology of Fishes. 45 (2): 177–190. doi:10.1007/BF00005232. ISSN 1573-5133. S2CID 1769706.
- Knott, Cheryl Denise; Emery Thompson, Melissa; Stumpf, Rebecca M.; McIntyre, Matthew H. (2010-01-07). "Female reproductive strategies in orangutans, evidence for female choice and counterstrategies to infanticide in a species with frequent sexual coercion". Proceedings of the Royal Society B: Biological Sciences. 277 (1678): 105–113. doi:10.1098/rspb.2009.1552. ISSN 0962-8452. PMC 2842634. PMID 19812079.
- Taborsky, Michael; Schütz, Dolores; Goffinet, Olivier; Doorn, G. Sander van (2018-05-01). "Alternative male morphs solve sperm performance/longevity trade-off in opposite directions". Science Advances. 4 (5): eaap8563. Bibcode:2018SciA....4.8563T. doi:10.1126/sciadv.aap8563. ISSN 2375-2548. PMC 5966226. PMID 29806019.
- Partridge, Charlyn (2017), "Sneak Copulation as an Alternative Mating Strategy", in Shackelford, Todd K.; Weekes-Shackelford, Viviana A. (eds.), Encyclopedia of Evolutionary Psychological Science, Springer International Publishing, pp. 1–3, doi:10.1007/978-3-319-16999-6_3610-1, ISBN 978-3-319-16999-6
- Oliveira, Rui F. (Rui Filipe Nunes Pais de) (2008). Alternative reproductive tactics an integrative approach. Cambridge University Press. pp. 1–21. ISBN 978-0-521-83243-4. OCLC 850824972.
- Gotthard, Karl; Berger, David; Bergman, Martin; Merilaita, Sami (2009-10-01). "The evolution of alternative morphs: density-dependent determination of larval colour dimorphism in a butterfly". Biological Journal of the Linnean Society. 98 (2): 256–266. doi:10.1111/j.1095-8312.2009.01290.x. ISSN 0024-4066.
- Fausto-Sterling, Anne; Coll, Cynthia Garcia; Lamarre, Meghan (2012-06-01). "Sexing the baby: Part 1 – What do we really know about sex differentiation in the first three years of life?". Social Science & Medicine. Gender and health: Relational, intersectional, and biosocial approaches. 74 (11): 1684–1692. doi:10.1016/j.socscimed.2011.05.051. ISSN 0277-9536. PMID 21802808.
- Bishop, KATHERINE M.; Wahlsten, DOUGLAS (1997-01-01). "Sex Differences in the Human Corpus Callosum: Myth or Reality?". Neuroscience & Biobehavioral Reviews. 21 (5): 581–601. doi:10.1016/S0149-7634(96)00049-8. ISSN 0149-7634. PMID 9353793. S2CID 9909395.
- Joel, Daphna; Berman, Zohar; Tavor, Ido; Wexler, Nadav; Gaber, Olga; Stein, Yaniv; Shefi, Nisan; Pool, Jared; Urchs, Sebastian; Margulies, Daniel S.; Liem, Franziskus (2015-11-30). "Sex beyond the genitalia: The human brain mosaic". Proceedings of the National Academy of Sciences. 112 (50): 15468–15473. Bibcode:2015PNAS..11215468J. doi:10.1073/pnas.1509654112. ISSN 0027-8424. PMC 4687544. PMID 26621705.
- Voigt, Cornelia; Gahr, Manfred (2011-06-08). "Social Status Affects the Degree of Sex Difference in the Songbird Brain". PLOS ONE. 6 (6): e20723. Bibcode:2011PLoSO...620723V. doi:10.1371/journal.pone.0020723. ISSN 1932-6203. PMC 3110770. PMID 21687671.
- Geary, David C. (2017). "Evolutionary framework for identifying sex- and species-specific vulnerabilities in brain development and functions". Journal of Neuroscience Research. 95 (1–2): 355–361. doi:10.1002/jnr.23794. ISSN 1097-4547. PMID 27870407.
Bibliography
- Baum, Michael J. (2006). "Mammalian animal models of psychosexual differentiation: When is 'translation' to the human situation possible?". Hormones and Behavior. 50 (4): 579–88. doi:10.1016/j.yhbeh.2006.06.003. PMID 16876166. S2CID 7465192.
- Crouch, RA (1998). "Betwixt and between: The past and future of intersexuality". The Journal of Clinical Ethics. 9 (4): 372–84. PMID 10029838.
- Hughes, I A; Houk, C; Ahmed, S. F.; Lee, P. A. (2005). "Consensus statement on management of intersex disorders". Archives of Disease in Childhood. 91 (7): 554–63. doi:10.1136/adc.2006.098319. PMC 2082839. PMID 16624884.
- Phoenix, C. H.; Goy, R. W.; Gerall, A. A.; Young, W. C. (1959). "Organizing Action of Prenatally Administered Testosterone Propionate on the Tissues Mediating Mating Behavior in the Female Guinea Pig". Endocrinology. 65 (3): 369–382. doi:10.1210/endo-65-3-369. PMID 14432658.
- Wallen, Kim (2005). "Hormonal influences on sexually differentiated behavior in nonhuman primates". Frontiers in Neuroendocrinology. 26 (1): 7–26. doi:10.1016/j.yfrne.2005.02.001. PMID 15862182. S2CID 10236292.
- Wilson, BE; Reiner, WG (1998). "Management of intersex: A shifting paradigm". The Journal of Clinical Ethics. 9 (4): 360–9. PMID 10029837.
External links
- Human Sexual Differentiation by P. C. Sizonenko
- The Ciba Collection of Medical Illustrations: Vol.2, Reproductive System by Frank H. Netter, M.D. comparing female and male reproductive systems development and anatomy
- Development of the Female Sexual & Reproductive Organs – illustrations comparing female and male genitalia during the early development